It is used to study and identify chemical substances or functional groups in solid, liquid, or gaseous forms. Infrared spectroscopy is based on molecular vibrations caused by the oscillation of molecular dipoles.
Interpreting Ir Specta: A Quick Guide – Master Organic Chemistry
The stuff in the fingerprint region, because there are so many possible groups that could contribute, is pretty hard to actually interpret.
Ir spectrum purity. The symmetrical stretch of co 2 is inactive in the ir because this vibration produces no change in the dipole moment of the molecule. We perform infrared vibrational analysis on small protonated water clusters h (+) (h (2)o) (n), with n = 2, [ellipsis (horizontal)], 6, at room temperature. You can create known percent solutions of the sample, take the irs, create a linear plot (beers law) and derive the equation of the slope to understand the relationship between peak area and concentration.
Infrared spectroscopy generally refers to the analysis of the interaction of. Light having a longer wavelength and a lower frequency than visible light. The near, mid, and far ir (see figure 15.2).
Infraredspectrometricpuritycontroloforganic liquidsandwater for the purpose of purity control, spectral comparisons are performed by regressing the infrared spectrum of a potentially polluted sample on the reference spectrum of the pure compound. A spectrum is obtained periodically, and a stored spectrum of the pure ester is digitally subtracted from that of the sample. Transmitance mary 1500 1000 100 ten shove and annotate it.
It is not a general rule because sometimes a very small signal in the ftir means an impurity that is very important or toxic. The mid ir region is of greatest practical use to the organic chemist. Infrared spectroscopy ( ir spectroscopy or vibrational spectroscopy) is the measurement of the interaction of infrared radiation with matter by absorption, emission, or reflection.
Bonds have characteristic vibrations depending on the atoms in the bond, the number of bonds and the orientation of those bonds with respect to the rest of the molecule. In order to be ir active, a vibration must cause a change in the dipole moment of the molecule. Then you can test your unknown to the calibration curve to find the percent concentration (units of ppm) and calculate the purity.
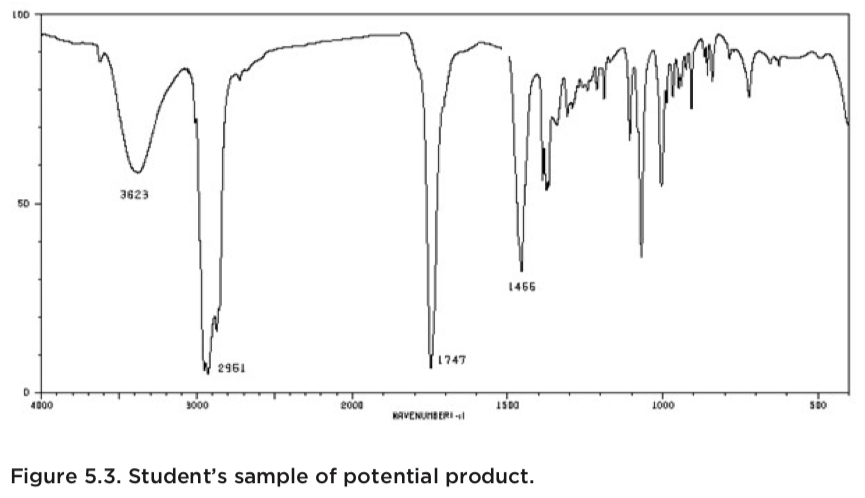
For example, an ir spectrum corresponding to a product with a depressed melting point will often show peaks that are not expected for a pure sample. Iii) the amide i peak at 1653 ± 5 cm −1 was fitted with lorentzian function. Stretching and bending vibrational modes for co2.
Therefore ir spectroscopy is widely used as \ ngerprint method for identifying unknown compounds. Let us look at a specific example. Bii u report table bo.1:
The instrument manufacturers normally provide proprietary software for this purpose. The full spectrum can only be viewed using a free account. A typical ir spectrum contains about 4000 data points and computer software is needed for processing the spectral data.
The ir region is divided into three regions: The sensitivity of a normal ftir are 100ppm. Ir spectroscopy (which is short for infrared spectroscopy) deals with the infrared region of the electromagnetic spectrum, i.e.
Therefore ir spectra are sometimes reported in µ This leaves only a spectrum of the impurities, which can be compared with a stored database of impurities. Chemists prefer to work with numbers which are easy to write;
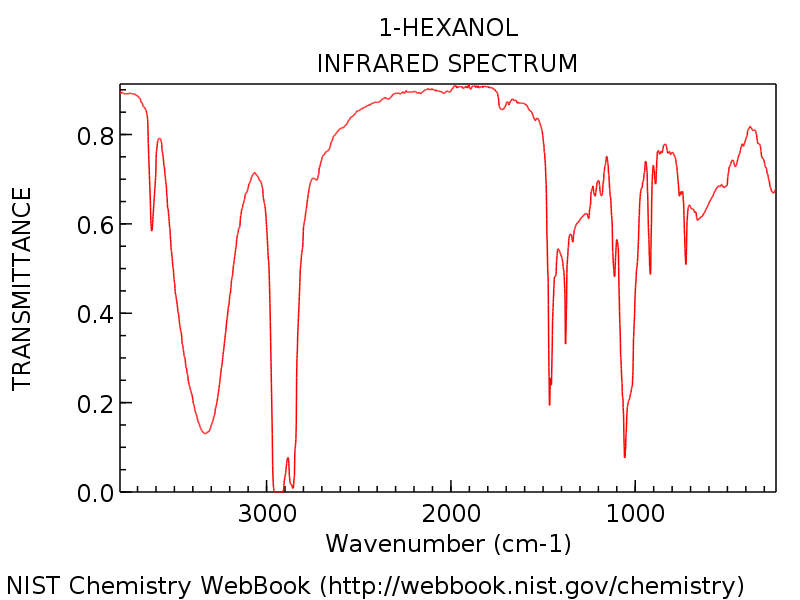
The computer identifies the impurities and automatically generates a report on the composition of the ester. The ir spectrum in the example is actually just the spectrum for ethanol.

Fourier Transform Infrared Spectrum Of Rhodamine B Purity Reference... | Download Scientific Diagram