Mar 13, 2018 0.34 m ≈ 2.05 ⋅ 1023 h 3o+ions per mol explanation: All solutions can be placed down the sink.
Acids And Bases: Ph, Kw, Weak Acids And Bases, And Buffers
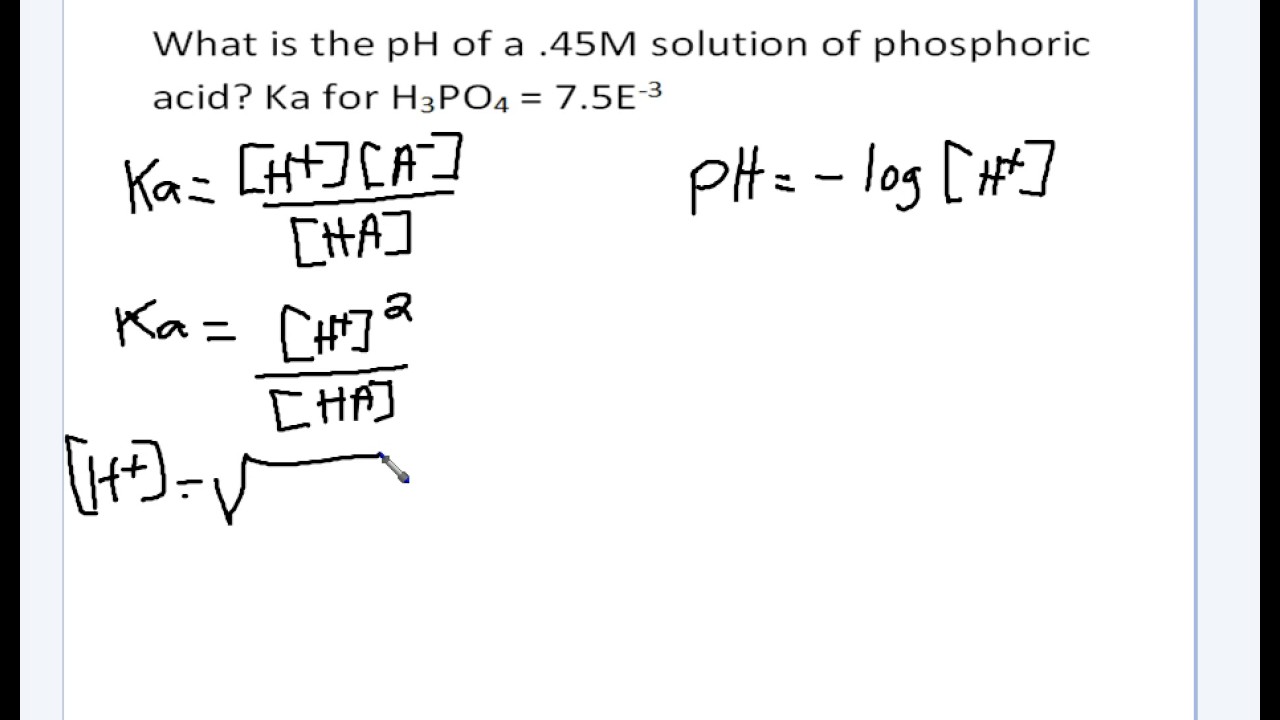
Follow if you have the concentration in molarity and ka, this allows you to calculate [h+].

How to find ka from molarity. Viewed 12k times 0 $\begingroup$. Molarity = 5 / (1.2 * 36.46) = 0.114 mol/l = 0.114 m. You can also use this molarity calculator to find the mass concentration or molar mass.
Modified 5 years, 2 months ago. As mass / volume = molarity * molar mass, then mass / (volume * molar mass) = molarity. That gives y = 0.0013 (assumption valid!) “e” represents “x10^” vote reply
After that, go for entering all the parameters in their designated fields and select their units from their corresponding drop down lists. Calculating concentration of h+ ions using ka value [closed] ask question asked 5 years, 2 months ago. Now tap the calculate button.
Solution for which you measured ph. If so can anyone walk through it? For example given an acid ha, and its ka:
To go from molarity to ph, use your calculator or a similar tool to take the logarithm to the base 10 (the default base) of the molarity, reverse the sign to get a positive value, and you're done! Return your unknown vial to your ta. About press copyright contact us creators advertise developers terms privacy policy & safety how youtube works test new features press copyright contact us creators.
Thu aug 04, 2011 8:53 pm has upvoted: With this information i should be able to do this question, but i'm stumped on how i can find this value. Substitute the known values to calculate the molarity:
This explains why the kb equation and the ka equation look similar. From these two values determine ka for the acid. Hence the more potent the acid will be.
Finding the value of [h^+] requires knowing the value of ka for the acid and the initial concentration of the solution. We use the ph equation, which states that ph = −log[h +] or ph = − log[h 3o+] it is the same equation anyways, as water can dissociate two ways. eqhno_ 2 (aq) + h_ 2o_ {.
Before you leave the lab, clean your buret with distilled water, and then place it upside down in the buret clamp with the stopcock open. Consider 0.10 m acetic acid, ch3cooh. From the first drop down list, do select which method you want to calculate ph with.
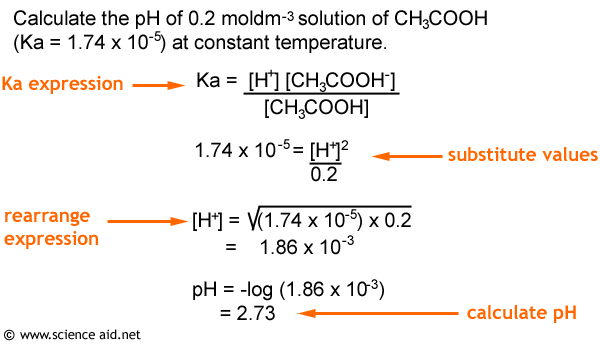
Calculate the ka value of a 0.021 m aqueous solution of nitrous acid ( hno2) with a ph of 3.28. We can use molarity to determine the ka value. Convert the expressions above to obtain a molarity formula.
Knut ritter master in chemistry upvoted by Write the balanced dissociation equation for the weak acid. Do you have any tips?
Pka is the criterion used to determine the acidity of the molecule. Finally, turn in your blue notebook pages. The lesser the pka is, the molecule would hold proton less tightly;
It is used to measure the acid strength. Ka = [product] / [reactant] we can fill the concentrations to write the ka equation based on the above reaction. After doing so, select the desired acid or base depending upon the selection you made.
This video shows how you can calculate the ka of an acid, if you're given the ph of the solution (and its concentration, of course). 2 posts • page 1 of 1. Can one calculate the ka value if the problem only gives the molarity of the solution?
Ka is the acid dissociation constant. The producted salt is base, so we use the formula: