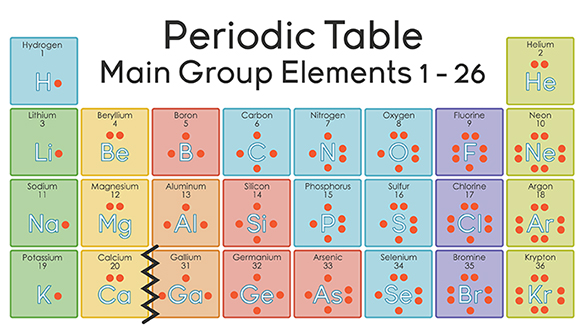
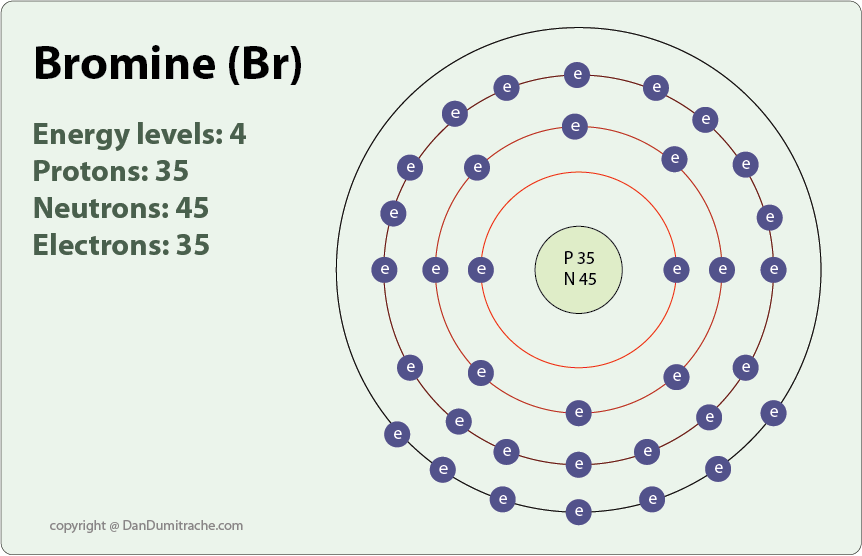
Bromine has an electron configuration of 1s22s22p63s23p64s23d104p5 the valence electrons are in the 4s and 4p orbitals giving bromine 7 valence electrons. The atomic number for bromine is 35, which means it has 35 protons in its atomic nuclei.
What Is The Number Of Valence Electrons In Nitrogen? | Socratic
Valence describes how easily an atom or a free radical can combine with other chemical species.
How many valence electrons in br. From the above electron configuration, it is clear that the valence electron is 7 because there are 7 number of electrons in outer shell. Therefore, the valence electrons of bromine are seven. How does br have 7 valence electrons?
Beside above, why does br have 7 valence electrons? Valence refers to the ability of an atom form bonds. Four five eight fourteen answer 5.0 /5 80
119 rows valence electrons in bromine (br) 7: Below is the lewis dot structure for a neutral bromine atom, which has. The terms “ oxidation degree ” and “ valence ” may not be the same, but they are numerically almost identical.
Valence electrons in rubidium (rb) 1: The atomic number of bromine is 35, which means it has 7 electrons in its valence shell. Valency of bromine (br) there are many different ways to find out the valency of an atom which reflects the ability of an atom to bond with other atoms.
Thus, bromine has seven valence electrons. Thus, the valence electron of bromine is v ebr =7 v e b r =. Now, the electron configuration of bromine shows that the last shell of bromine has seven electrons.
The first is to use the periodic table to figure out how many electrons bromine has in its. A neutral bromine atom would also have 35 electrons. Br has 7 valence electrons and h has 1 valence electron, so that's 8 in total.
The electronic configuration of bromine is 1s22s22p63s23p64s23d104p5 and the valence electrons are in the 4s and 4p orbitals giving bromine 7 valence electrons. The conditional charge of an atom’s atom is called the oxidation state. It can be either positive or negative.
Valence electrons in krypton (kr) 8: Valence electrons in strontium (sr) 2: 12 electrons explain why conduction band electrons are typically found at the bottom of the conduction band whereas holes are found at the top of the valence band.
Valence electrons found in the s and p orbitals of the highest energy. You need 2 electrons for a bond so you end up with h bonded to br and 6 electrons around br. Another way to find the valence electrons is by the group number the atom is found in.br is in group 7a of the periodic table, which means it has 7 valence electrons (as all other atoms in the same group).
So, it is possible to determine the properties of bromine from the electron configuration. 21,481 views sep 2, 2020 there are two ways to find the number of valence electrons in bromine (br). One two five seven how many valence electrons are available for bonding in silicon?
Periodic table with valence electrons labeled (7 hd images) periodic table with charges labeled on it (7 hd images) electronegativity chart of all elements. The total number of electrons in a bromine atom is therefore 35. How many valence electrons are available for bonding in bromine (br)?
Why Does Fluorine Have A Higher Ionization Energy Than Bromine? | Socratic

Bromine Bohr Model - How To Draw Bohr Diagram For Bromine (Br) Atom