2 in the 5s, 5 in the 5p and up to 10 in the 4d, although it only needs to use 4 of its 4d electrons to do the bonding in this molecule. Since the 4d subshell contains a total of 5 orbitals, and since each orbital can hold a maximum of 2 electrons of opposite spins, you can write a total of 10 quantum number sets for.
Shells And Subshells - A-Level Chemistry
Click to see full answer accordingly, how many electrons can 4d hold?

How many electrons can 4d hold. So, iodine can use all of these electrons in chemical bonding: As each orbital can hold maximum two electrons so total electrons p orbital can hold is 6. How many electrons can fill 4d?
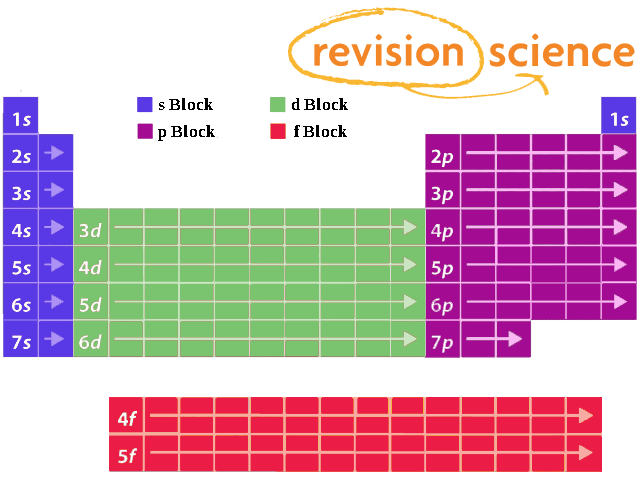
Secondly, how many electrons does each sublevel hold? So a total 14 electrons are present in a 4f orbital. The 3d, 4d etc., can each hold ten electrons, because they each have five orbitals, and each orbital can hold two electrons (5*2=10).feb 20, 2014.
The pauli exclusion principle (wolfgang pauli, nobel prize 1945) states that no two electrons in the same atom can have identical values for all four of their quantum. So in short, the 4d orbitals (and any set of d orbitals) can hold 10 electrons, and the 4f orbitals (and the 5f orbitals) can hold 14 electrons. Therefore the 4p orbital can hold two electrons and the 4p subshell can hold a total of six.
Each orbital can contain two electrons which are present in opposite directions. Enjoy :) 5.4k views view upvotes ali abdulla. How many electrons can 4s hold?
1 i think of subshells as the building of electrons sets: The s sublevel has just one orbital,.

Electrons. - Ppt Video Online Download
How Many Electrons Can The Fourth Energy Level Hold According To Niels Bohr? | Socratic