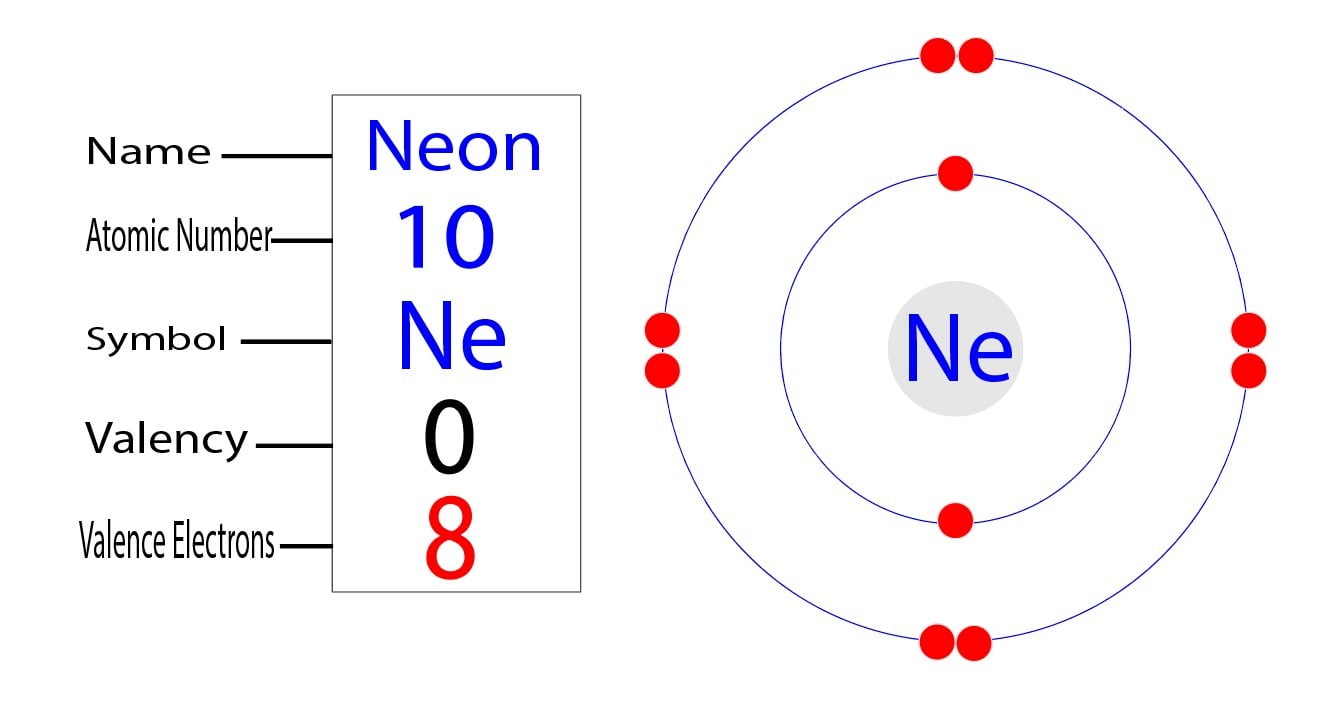
Therefore, a neon atom will have two electrons in the first shell and eight in the 2nd shell. The atomic number of neon is z=10.
Neon Has 10 Electrons. How Many Electrons Will Be Orbiting In The Outermost Shell, Farthest Away From The Nucleus? - Quora
Neon has a closed shell configuration, and.
How many electrons are in neon. In writing the electron configuration for neon the first two electrons will go in the 1s orbital. So, since neon is an inert nobel gas, the number of electrons in its outermost electron shell is eight. In bohr's model, for unielectronic atoms, the following symbols are used :
Again another two electrons will go in 2s orbital and the remaining 6 will go to the 2p orbital. The remaining six electrons will go in the 2p orbital. 7 protons, 7 electrons, 7 neutrons oxygen, o 8 protons, 8 electrons, 8 neutrons fluorine, f 9 protons, 9 electrons, 10 neutrons neon, ne 10 protons, 10 electrons, 10 neutrons sodium, na 11 protons, 11 electrons, 12 neutrons magnesium, mg 12 protons, 12 electrons, 12 neutrons aluminium, al 13 protons,.
The neutral atom of neon has 10 electrons. If an ion has a 2+ charge, like zn 2+, this means there are two more protons than electrons. So the number of electrons should also be 10.
However, the number of neutrons can vary depending on the isotope. The first two electrons will go in the 1s orbital as the s orbital can hold a maximum of 2 electrons only. An isotope of neon is a specific type of neon.
There are 10 electrons in a single atom. How many valence electrons are in an atom of neon?. Its octet is already complete and therefore, it does not require to undergo any other chemical reaction.
How many unpaired electrons are in the neon atom? Neon is a non metal element. Neon atom electron configuration (bohr model) the atomic number of neon is 10.
U n,z→ potential energy of e −;k n,z→ kinetic energy of e −; There are 10 electrons in a single atom. How many valence electrons are in an atom of neon?.
Therefore, the order of the number of electrons in each shell of the neon (ne) atom is 2, 8. Now we know each orbital contains 2 electrons with spins opposite to each other so half electrons will have one type (clockwise) of spin and other. Neon is the tenth element with a total of 10 electrons.
1s²2s²2p6 all the orbitals will be filled. The electron configuration for neon (ne), shows that there are eight electrons in the l shell, and two in the k shell. The n= 2 shell can be occupied by 8 electrons and in case of neon, all the available electronic states are occupied, i.e.
R n,z→ radius of n th orbit with atomic number z; Also, it is present in group viii, so that should tell you anyway that it has eight valence electrons. How many electrons are in a neon atom a 9 b 10 c 20 d.
That is, the number of electrons in neon is 10. The neon atom has a total of 10 electrons so, we have to put 10 electrons in orbitals. The electronic configuration of neon will be :
Pages 23 ratings 100% (1) 1 out of 1 people found this document helpful; How many electrons in the neon? How many electrons in the neon?
There are 10 electrons in a single atom. How many electrons are in a neon atom a 9 b 10 c 20 d 21 e none of the above. The electronic configuration of a neon atom is:
How many protons neutrons and electrons are in each element? Neon is a non metal element. This means that the first shell of neon contains two electrons and the second shell has eight.
Since 1s can only hold two electrons the next 2 electrons for ne go in the 2s orbital.

Valence Electron: Definition, Configuration & Example - Video & Lesson Transcript | Study.com