Beryllium atom has 4 electrons. We know that each beryllium atom has four electrons.
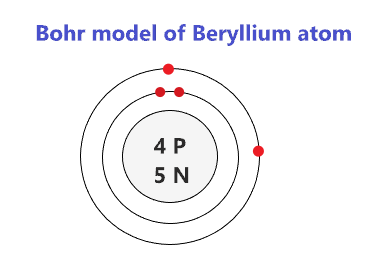
Beryllium Bohr Model - How To Draw Bohr Diagram For Beryllium(Be) Atom
Giribabu musalagari electronics engineer (26 years);
How many electrons are in beryllium. Teacher (9 years) author has 3.3k answers and 1m answer views aug 5 related How many unpaired electrons are in the beryllium atom? Comments (1) reply and earn points.
Neutron number and mass number of beryllium mass numbers of typical isotopes of beryllium are 9. This is how because 2 valence electrons are located in the 2s subshell. And so, its valency will be 2 because it has 2 electrons in its outermost shell.
Notable gemstones high in beryllium include beryl (aquamarine, emerald) and chrysoberyl.it is a relatively rare element in the universe,. In this sense the third shell can hold 8 electrons. In the case of beryllium, there are 4 electrons that are present in the 2 orbits of its atom.
And these electrons give a +2 oxidation and their ability is to form the 2 covalent bonds. Now, as we can see that the number of valence electrons in beryllium is 2. The full electron configuration of beryllium is 1s2 2s2.
We already know the beryllium atomic number is 4. 4s2 not the third shell but the next 10 electrons go into the 3d orbitals that are part of the third shell but shown on the fourth shell level. So the third shell can be considered to hold 8 or 18 electrons but in total the third shell can hold 18 electrons.
In this video we’ll use the periodic table and a few simple rules to find the number of protons and electrons for the beryllium ion (be2+). The electronic configuration of beryllium is: Firstly, 2 electrons are placed at the first orbital which is 1s.
The chemical symbol for beryllium is be. So, the electronic configuration of be will be 1s² 2s². The electron structure of beryllium indicates that there are two electrons within the k shell and two inside the l shell.
Secondly, 2 electrons are placed at the second orbital which is 2s. This means that the first shell of beryllium contains two electrons, while the second shell contains two. 1s 2 2s 2 or can also be represented as [he] 2s 2.
How many electrons are in the highest occupied energy level of beryllium? Beryllium the solid form is metallic. From the periodic table we can find the element symbol,.
One beryllium atom has four (4) electrons, four (4) protons, and three (3), five (5) or six (6) neutrons, depending on the isotope being considered. To see other 2 answers. How many unpaired electrons are in the titanium atom?
Also, check here the hydrogen electron configuration what is. This means its electron configuration will be 1s 2, 2s 2. Beryllium is a chemical element with the symbol be and atomic number 4.
As the s sublevel in each electron. Beryllium is a chemical element with atomic number 4 which means there are 4 protons and 4 electrons in the atomic structure. What is the number of valence electrons in beryllium
Neutral, isolated and unexcited beryllium atoms have two electrons in their highest energy level. As beryllium has an atomic number of four, it will also have four electrons if it is a neutral atom.
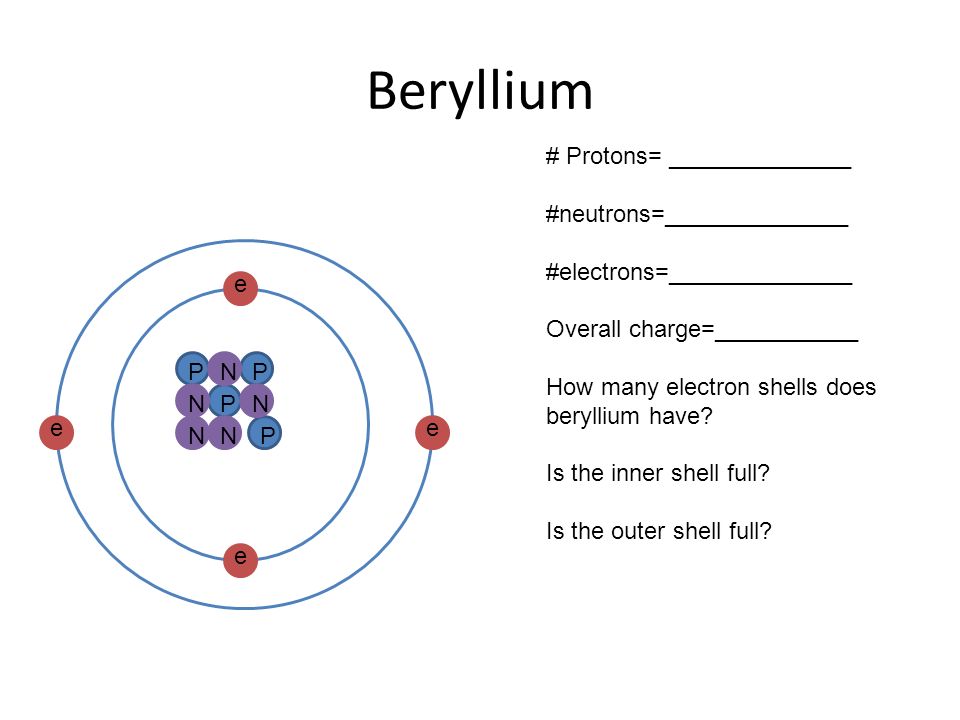
Interpreting Atomic Structure - Ppt Video Online Download
Interesting Facts/Atomic Structure Of Beryllium - Beryllium The Best!