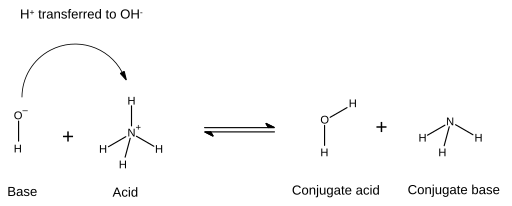
Therefore, in this process for the reaction to occur, h2o acts as the base while h3o+ acts as its conjugate acid. On the other hand, a conjugate base is what is left over after an acid has donated a proton during a chemical reaction.
So i am thinking that the conjugate acid is h x 2 o.

H2o base conjugate acid. What is the conjugate base of h2po 4? A oh − and f −, respectively b h 3o and f − , respectively c h 3o + and f − , respectively d h 3o + and h 2f +, respectively medium neet solution verified by toppr correct option is a) a conjugate base formed after releasing h + ions so, for given acids h 2o and hf conjugate base would be Let us take the example of bicarbonate ions reacting with water to create carbonic acid and hydronium ions.
Are h2co3 hco3 conjugate pair? Conjugate base for bronsted acids h 2o and hf are: N a o h n a x + + o h x −
H2po4 (aq) + h2s(aq) = h3po4(aq) + hs (aq) d. Question (d) ph and poh add up to 14 in an aqueous solution, so we can calculate ph as follows:. Clo2 how in the world do you determine which one is the conjugate acid and which one is the conjugate base?
How do you write a conjugate base for h3o positive? The conjugate acid of water is represented as h3o+. \ (\ce nh4+\) is the slightly stronger acid (ka for \ (\ce nh4+\) = 5.6 × 10−10).
So, (4.) h2o h 2 o is the conjugate acid. In the reactant side, hydrogen phosphate (h2po4) is an acid while h2o is amphiprotic, meaning it can be a base or acid. A conjugate acid is formed when the base accepts a proton.
And that's exactly what it does in the reverse reaction. On the other hand, a conjugate base is merely what is left after an acid has donated a proton in a chemical reaction. [cr (h2o)6]3++h2o⇌ [cr (h2o)5 (oh)]2++h3o+ show transcribed image text expert answer 100% (1 rating) (i hope you like.
This is a bronsted question. Hco₃⁻ + h₂o → h₂co₃ + oh⁻ base + acid → conj a + conj b we see that hco₃⁻ becomes h₂co₃. Stronger acids form weaker conjugate bases, and weaker acids form stronger conjugate bases.
View acids and bases_02_09_21.pdf from chem 102 at los angeles city college. However, wouldn't that mean that the conjugate acid of any base of the form ( s o m e t h i n g) o h + h x + ( s o m e t h i n g) x + + h x 2 o would be water, and that seems unsettling to me. And that means that fluoride has to be acting as the base.
Hf (aq) + h2o) = h2o (aq) + f(a (aq) this problem has been solved! Answer to solved label the acid, base, conjugate acid, and conjugate. What is the chemical formula for the conjugate base of water?
H3o+ is the conjugate acid of h2o since it can lose a proton in the reverse reaction. Identify the acid, base, conjugate acid, and conjugate base in the following reactions. Hco3 (aq) + oh(aq) = co3 (aq) + h2o(1) c.
To formulate the conjugate acid/conjugate base we simply add or subtract a proton h+ and conserve charge. Well i'm glad you asked. I also believe that since n a o h undergoes the following reaction: