Rank their conjugate acids, hx, hy, and hz, in order of decreasing strength. It is the conjugate base of h2so4.

What Is The Conjugate Acid Of Hso4-? - Youtube
H2so4 is the chemical name for sulfuric acid, and the conjugate base is hydrogen sulfate.
Conjugate base of hso4. Even though hso4 is also an acid, it can either be a conjugate acid or a conjugate base depending on context. The university of waterloo science page lists hso4 as the conjugate base of h2so4. Acid conjugate base base conjugate acid hcio2 а.
That is because sulfuric acid is a strong acid and completely disassociates in water. Express your answer as a chemical formula. Express your answer as a chemical formula.
What is the conjugate base of hso 4−? What is the formula for the conjugate base of hso− 4 h s o 4 −? Medium solution verified by toppr although it has a negative charge, it will never accept a h + to form h 2so 4(sulfuric acid).
A conjugate base is not necessarily a basic molecule. Conjugate bases are defined as molecules that are formed when an acid loses a hydrogen ion. The species that donates a hydrogen cation or proton in a reaction is a conjugate acid, while the remaining portion or the one that accepts a proton or hydrogen is the conjugate base.
As the acid releases a proton or. When an acid releases a proton the residue must be a base. Hence, a conjugate base is a species formed by the removal of a proton from an acid.
Part d among three bases, x−, y−, and z−, the strongest one is y−, and the weakest one is z−. The way we would be able to identify a conjugate base is if it has only one proton was removed from it in the acidic form. For conjugate base, remove an h+ from the acid, so the conj.
The conjugate base means it has to add h proton back in backward reaction. Conjugate base in a reaction of an acid in an aqueous solution, it loses a proton or hydrogen ion. This is called the conjugate base of the…
Is the sulfate ion the conjugate base of sulphuric acid? Therefore, option a is correct. So, we call a − to be the conjugate base of the acid ha.
Therefore, the sulfate ion (so 42−) is the conjugate base of hso 4− was this answer helpful? H2so4 is sulphuric acid, a very strong acid. Even though hso4 is also an acid, it can either be a conjugate acid or a conjugate base depending on context.
H2so4 is the chemical name for sulfuric acid, and the conjugate base is hydrogen sulfate. Hso4 is hydrogen sulphate and an amphiprotic species. Part b what is the conjugate base of hso4−?
Top 6 posts • page 1 of 1.
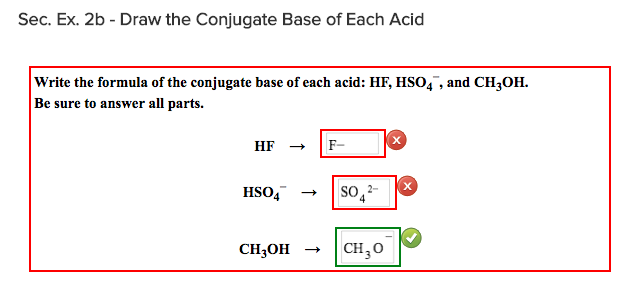
Solved Sec. Ex. 2B - Draw The Conjugate Base Of Each Acid | Chegg.com
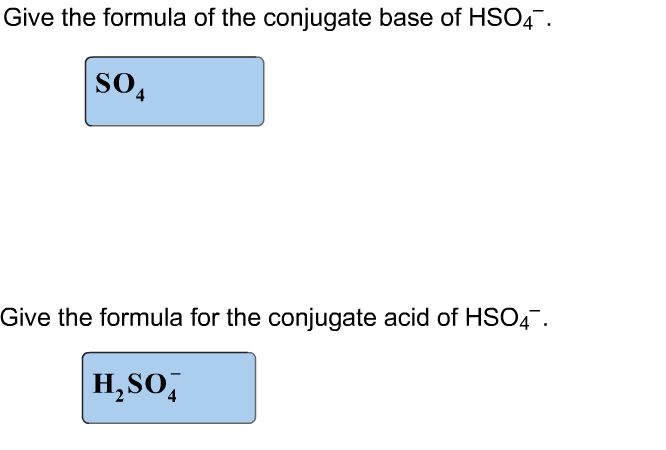
Solved Give The Formula Of The Conjugate Base Of Hs04 So 4 | Chegg.com