Write the formula of the conjugate base for acid hclo2. Which of the following would you expect to be the strongest acid?
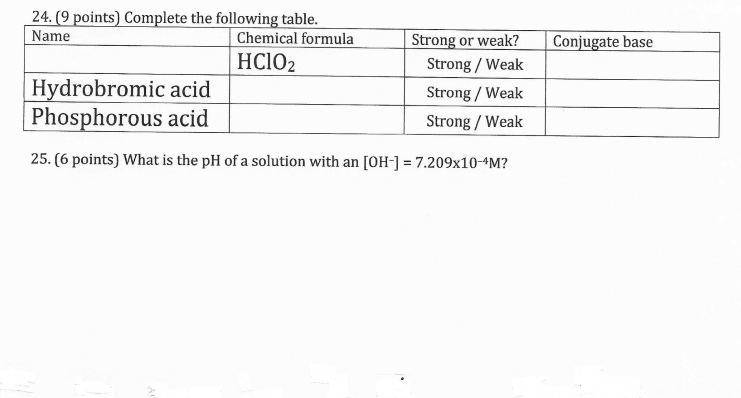
Solved Conjugate Base 24. (9 Points) Complete The Following | Chegg.com
To find the conjugate acid there are a few steps:
Conjugate acid for hclo2. Hio2 hbro2 hclo2 hclo hio a: The pure substance is unstable, disproportionating to hypochlorous acid (cl oxidation state +1) and chloric acid (cl oxidation state +5): It is a weak acid.
The central atom being chloride ion. You further know that a base accepts a proton, and an acid donates a proton. Write the formula of the conjugate base for acid h3po4.
Well i'm glad you asked. In this case your conjugate acid is h3po4. If a proton is less strongly attached to any one of the oxygens, then you get a stronger acid.
Explanation a) nh3 is a base which means it has capacity to accept the hydogen ion so if it accepts a hydrgen ion it will become nh4+ ion which is an acid since it now can donate a hydrogen ion but it a conjugate acid to base nh3 since it is. Write the formula of the conjugate base for acid hbro. You know that the conjugate base of an acid is the anion after protonation of water.
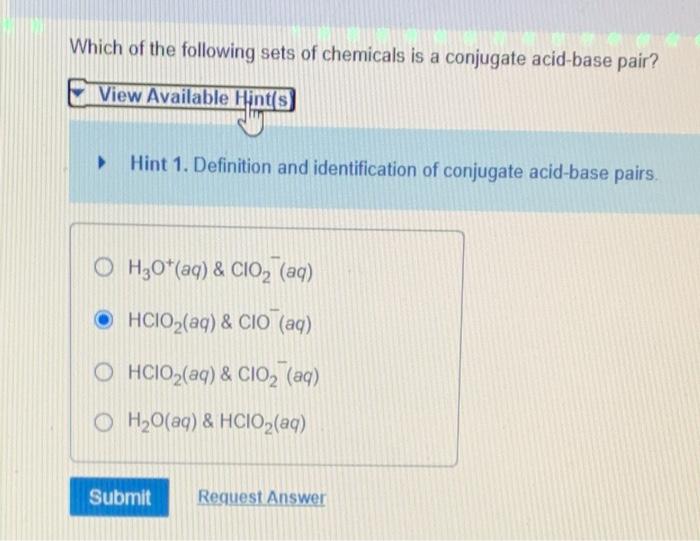
The conjugate base is the substance formed after an acid loses a proton. 2 hclo 2 → hclo + hclo 3 The formula is be (clo2)2.
Write the formula of the conjugate base for acid hcn. Chlorous acid chlorous acid is an inorganic compound with the formula hclo 2. Let us take the example of bicarbonate ions reacting with water to create carbonic acid and hydronium ions.
Express your answer a chemical formula. What is the chemical equation for hclo2? The conjugate base of a strong acid is neutral.
Chlorous acid is a weak acid and this acid has an oxidation state of +3. To find the formula of a conjugate base subtract 1 h+. The central atom has 8 valence electrons.
Write the formula of the conjugate base to the following species regarded as an acid: When we add hclo2 to h2o the hclo2 will dissociate and break into h+ an. That is, 3s2 3p6 configuration in ground state.
Select the statements that correctly describe buffers.? So what is the oxidation state of chlorine in this chlorous acid? In this video we will look at the equation for hclo2 + h2o and write the products.
Na+ is the conjugate acid of a strong base, naoh. The oxidation number for cl in hclo is +1, +3 for hclo2, +5 for hclo3, and +7 for hclo4. H clo2 + h 2o ⇌ clo− 2 +h 3o+ explanation:
Hclo2 is called halous acid with chlorine having +3 oxidation state. Express your answer as a chemical formula. Hclo2 is the chemical formula (not equation) of the chlorous acid.
As the number of oxygens increases as you go from hclo to hclo4, the oxidation number of cl increases. To find the formula of a conjugate acid add 1 h+. What is the conjugate base of ch3co2h?
What is the conjugate base of hclo2 a: Chlorine has oxidation state +3 in this acid. The formula of chlorous acid is hclo2 h c l o 2.
This problem has been solved! Chlorous acid the formula of the conjugate acid for chlorite ion is chlorous acid. Calculate the pka of hypochlorous acid.
Hclo3 (chloric acid) is a stronger acid than hclo2 (chlorous acid) for the following reason. Each oxygen atom need 1 electron pair to complete the octet. 1) the ph of a buffer solution does not change significantly when any amount of a strong acid is added.
Clo2 how in the world do you determine which one is the conjugate acid and which one is the conjugate base? What is the conjugate acid of clo2 −? The ph of a 0.015 m solution of hypochlorous acid has a ph… a:
Hco₃⁻ + h₂o → h₂co₃ + oh⁻ base + acid → conj a + conj b we see that hco₃⁻ becomes h₂co₃. To make a formula for beryllium chlorite, the charges must be balanced at 0. Express your answer as a chemical formula.

