It doesn't form an octet by doing so thus it can take two more electrons by forming a coordinate bond to become an octet and thus it behaves as lewis acid. Salts are composed of related numbers of cations (positively charged ions).
15.2: Lewis Acids And Bases - Chemistry Libretexts
Its electronic configuration can be represented as follows:

Cl- lewis acid or base. This is because chlorine has. So water can be considered a lewis acid relative to chloride ion because h x 2 o hydrates chloride ions by hydrogen bonding. C l = 1 s 2 2 s 2 2 p 6 3 s 2 3 p 5 as the valence shell of chlorine consists of seven electrons, so it generally exists as a chloride ion by.
In the hclo3 lewis structure, a total of 7 lone pairs. Usually, we condsider both electronic. (lewis acid is defined as.
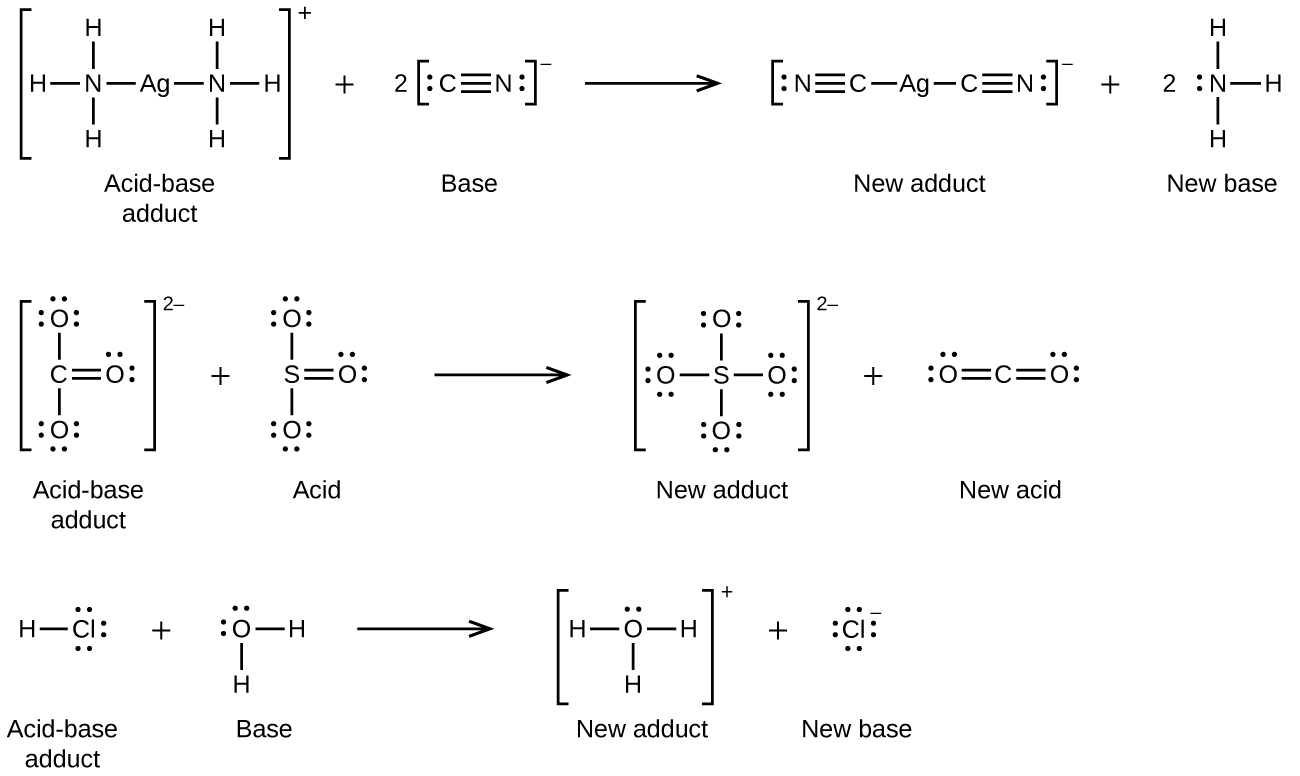
Since lewis acidity is tangled up with lewis structures, perhaps it is not too surprising that the concept has some serious problems. Yes is cl minus an acid or base? This states that a lewis base is a nucleophile.
Having three flourine surrounding one chlorine leaves two electron pairs that's available to be donated. The water lewis base wins and the proton transfers. Chemistry acids and bases lewis acids and bases 2 answers monzur r.
Hclo3 is a strong acid and it completely dissociates into the ions in solution. To make it simple, it is a substance through which a pair of electrons is donated to form a covalent bond. Nh3 is a lewis base.
A lewis acid is an acid which accepts an electron pair from a compound donor. In chemistry, neutralization or neutralisation (see spelling differences), is a chemical reaction in which an acid and a base react quantitatively with each other. Classify the following species into lewis acids and lewis bases.
Fecl 3 are lewis acids. Bf3 is not a lewis base because it has an empty p orbital, this is a lewis. Hcl is a lewis acid.
The reason is stated below, in the above image cited,. The molecular geometry of hclo3 is trigonal pyramidal. A lewis base is something that donates a pair of electrons.
Lewis' theory used electrons instead of proton transfer and specifically stated that an acid is a species that accepts an electron pair while a base donates an electron pair. In chemistry, a salt is an ionic compound that can be formed by the neutralization reaction of an acid and a base. Some molecules can act as either lewis acids or lewis bases;
May 22, 2018 cl− is a lewis base because it donates a nonbonding electron pair. And water is a lewis base relative to metal. Is cl a lewis acid or lewis base?

Ppt - Lewis Acids, Lewis Bases, And Curvy Arrows Powerpoint Presentation - Id:3936485
Is The Following Acid-Base Reaction Arrhenius, Bronsted-Lowry, Or Lewis: Alcl3 + Cl --> Alcl4- | Socratic