It is a has s p hybridisation, which yields a linear molecule. Get instant solutions when in doubt download our app.
Solved Consider The Resonance Structures For The Carbonate | Chegg.com
For example, in diatomic nitrogen, n≡n, the bond order is 3;

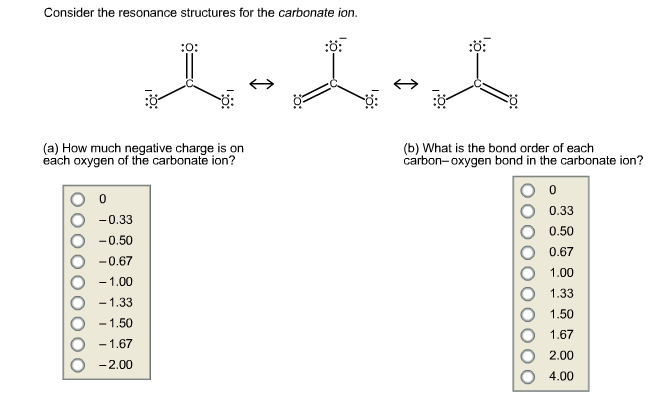
Bond order carbonate. Because of the linear symmetry around the central carbon, there is no net dipole moment, even though there is an electronegativity between oxygen and carbon. Bond order of polyatomic molecules can be determined using the resonating structures. Bond order is defined as the number of covalent bonds between two atoms in a molecule.
It has a bond order of 2, with a sigma bond and a pi bond. Then the value of (x+y) will be. You add up the total number of bonding pairs and divide by the total number of bonds.
That means you can draw these three resonance structures, where each bond in 2 out of 3 has an order of 1, and in the third there's a double bond. The values of bond order are a general indication of the number of bonds between two or more atoms; One double bond (2 electron pairs) and two single bonds (1 + 1= 2 electron pairs).
And before you go, check out this cell emf calculator! Bond order = number of bonds / number of groups = 4 / 3 = 1.33. Y , the value of (x + y) will be.
The bond order is therefore 4/3 = 1.33. Bond order is the number of chemical bonds between a pair of atoms and indicates the stability of a bond. Come back to our bond order calculator whenever you need a quick reminder on how to calculate bond order.
Co molecule co by default has a triple bond, so by chemical intuition, its bond order is 3. While bond order calculations are easy, finding bonding and antibonding electrons might get tricky! If the bond order of carbonate ion ( co3−2 c o 3 − 2) is expressed by the simple ratio x:y.
Bond order can be used to determine stability and strength of the bond. Class 11 >> chemistry >> chemical bonding and molecular structure >> bond parameters >> if the bond order of carbonate ion (co^2 question The formula mentioned to measure bond order is equal to one half of the difference between the number of electrons in the bonding and antibonding molecular orbitals.
In carbon monoxide, the bond between the atoms carbon and oxygen is a triple bond. Bond order and bond length indicate the type and strength of covalent bonds between atoms. C o 2 is a covalently bonded molecule.
It should be noted that the bond orders in the two theories do not always agree. Number bond pairs, bp (according to vsepr theory) = number of atoms directly attached to central atom; Calcium carbonate 780mg magnesium carbonate 130mg sodium.
Y , the value of (x + y) will be. (4 bonds averaged over three structures.) B = number of electrons in antibonding molecular orbitals.
The number of bond groups between individual atoms is 3. This can be verified by the usual equation: Class 11 >> chemistry >> chemical bonding and molecular structure >> bond parameters >> if the bond order of carbonate ion (co^2 question

