The green colour during fireworks and flares is produced by barium nitrate (it produces green flames).; Barium nitrate | ba(no3)2 or ban2o6.
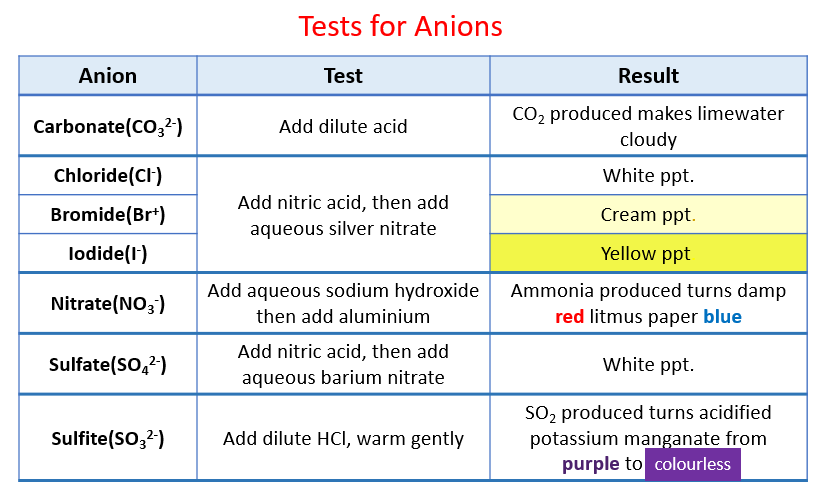
Chemical Analysis - Igcse Chemistry (Solutions, Examples, Worksheets, Videos)
White precipitate green precipitate(ii) to the second portion of solution add excess aqueousammonia.

Barium nitrate cation and anion. Barium sulphate is an insoluble salt and forms as a white precipitate. Identify the cation and anion for the following ionic compounds. It is a strong oxidant.
2ba(no 3) 2 → 2bao + 4no 2 + o 2; Iron, barium, calcium, lead and copper ions present in the sample need to be determined by using a series of tests. Then, add iron (ii) sulphate solution.
When barium chloride dissociates, it produces : (iii) to the third portion of solution, add an equal volume of aqueous sodium hydroxide. Iron (ii) sulphate solution, dilute sulphuric acid and concentrated sulphuric acid.
A metal reacts with a nonmetal to form an. The presence of cations and anions can be determined by a sequence of logical and chemical tests. Gc bbq near me call location tracker app tech hotels in yosemite valley 738.
Rough cut lumber lancaster pa. Nitric acid and silver nitrate solution. Ba(no 3) 2 decomposes to barium oxide (bao) at higher temperatures:
It’s important to follow a sequence so that certain ions can be eliminated in the process. The hydrochloric acid added is to prevent precipitation of barium carbonate. Carefully add concentrated sulphuric acid down the side of the test tube.
(i) to the first portion add dilute hydrochloric acid and about 1 cm3 of aqueous barium nitrate. Video answer:a compound is a substance that contains atoms of two or more different elements and these atoms are chemically joined together. Green precipitate paper turned bluewarm the mixturegently.
For example with naoh, many metals give a white ppt., and hence one can't say that the formation of a. What we have is potassium chloride. So we were taking a look at calcium nitrate, which has the formula c.
Be sure to include the correct charge! The electrostatic attraction between the positives and negatives brings the particles together and creates an ionic compound, such as sodium chloride. Amit guptahow to find anion and cation in a given salthow to analyse the given salt for acidic and basic radicalssalt analysis|| barium nitrate||ba(no3)2.
In other words, write the cation. 140mm to 100mm reducer all movie screencaps. Other barium compounds can be synthesized using barium dinitrate.
· cations occupy space between two anions (interstitial space. Hydrochloric acid and barium chloride solution. One type of reaction is not enough, to confirm the presence of barium, since other metal salts may give the same results.
Barium Nitrate | Ba(No3)2 - Pubchem

Question Video: Determining The Confirmatory Test For An Anion In An Unknown Salt | Nagwa