Be notified when an answer is. Why is boron 11 more abundant.

Solved:boron-10 And Boron-11 Are The Naturally Occurring Isotopes Of Elemental Boron. If Boron Has An Atomic Mass Of 10.81 Amu, Which Isotope Occurs In Greater Abundance?
It is both naturally occurring and a produced by fission.

Why is boron 11 more abundant. Chemistry matter isotopes 1 answer stephen x. Boron is identified as atoms containing five protons in the nucleus. The atomic mass of boron is 10.81 u.
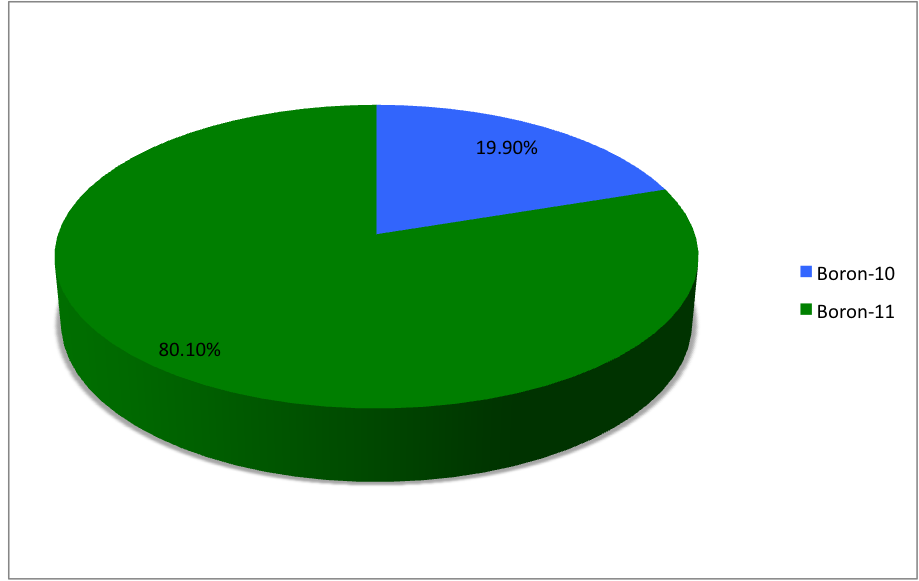
Which isotope of boron is more abundant and why?. The stable isotope of boron with relative atomic mass 11.009306, 80.1 atom percent natural abundance and nuclear spin 3/2. Which is more abundant, given that the atomic m of boron is 10.81 amu?
The boron atom is small with only. Lithium, beryllium and boron are unusually low. Slight variations in this proportion produce a range of.
Which boron isotope is more abundant. How many neutrons are in an. Which is more abundant, given that the atomic.
Posted by unknown at 10:01 pm. The atomic mass of boron is 10.81 u. Based on the atomic mass, which isotope should be more abundant?
Boron 11 metal is one of over 250 stable metallic isotopes.
Solved Question 50 (2 Points) There Are Two Stable Isotopes | Chegg.com