Bromine is a chemical element with atomic number 35 which means there are 35 protons and 35 electrons in the atomic structure. A) 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p4 b) 1s2 2s2 2p6 3s2 3p6 4d10 4s1 4p6 c) 1s2 2s2 2p6 3s2 3p6 4d10 4s2 4p5 d) 1s2 2s2.
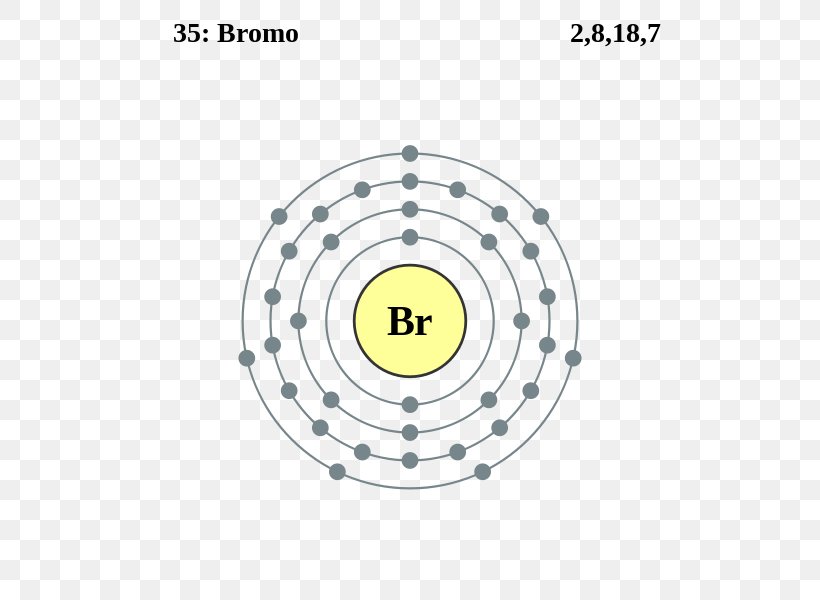
Electron Configuration Bromine Chemical Element Electron Shell Bohr Model, Png, 558X600Px, Electron Configuration, Area, Atom, Atomic
When hydrogen and bromine react, the bromine atom 'steals' the hydrogen atom's only electron.
What's the electron configuration for bromine. The bromine electron configuration is 4s23d104p5. Electron affinity of bromine is 324.6 kj/mol. In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as:
The ground state electron configuration of ground state gaseous neutral bromine is [ar]. The chemical symbol for bromine is br. 1s22s2 what is the orbital notation for bromine?
Selenium ← bromine → krypton. Full electron configuration of bromine: What is the unabbreviated electron configuration of bromine?
The arrangement of electrons in an atom by a superscript, in each sublevel is known as electron configuration. The majority of exposures to bromine occur by. The hydrogen atom then has no electrons and the bromine atom has 8 valence.
What’s the chemical formula for bromine? 4p5 and the term symbol is 2p3/2. 100% (1 rating) transcribed image text:
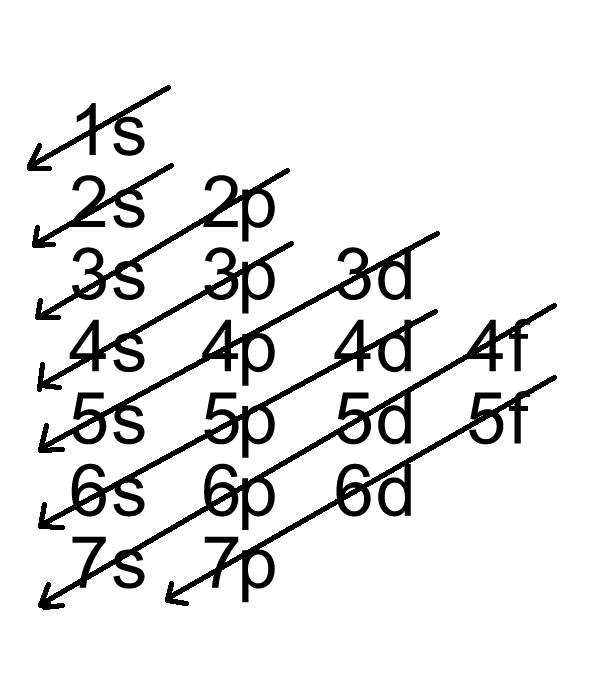
What is the last electron configuration. What is the electron configuration for bromine? There are 35 arrows in the electron configuration for bromine, which is for orbital filling.
The change in energy (in kj/mole) of a. See the answer what is the electron configuration for bromine? A bromine atom in the excited state could have an electron configuration of a.
These 35 arrows of bromine are only due to the atomic number of bromine. Electron configuration of bromine is [ar] 3d10 4s2 4p5. Question what is the electron configuration for bromine?
1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p5. Most common application of bromine bromine is intermediate in reactivity between chlorine. Medium solution verified by toppr all you need to do is work your way across the periodic table filling the orbitals as you go the full.

Dublin Schools - Lesson : Valence Electrons And Element Dot Diagrams