As the ka decreases, what will happen to the pka? (b) the boiling point decreases while vapor pressure increases.
Conductivity always decreases with decrease in concentration both, for weak and strong electrolytes.
What decreases he concentration of a solution. The concentration of a solute decreases with the increase in the volume of the solution. As we dilute a solution, the amount of hydronium ion per volume of. Thus, the boiling point increases while vapor pressure decreases.
Increasing the solvent would decrease the concentration. In a galvanic cell, when we add ions of the oxidation agent (in this example, zn2+) to the anode solution, it decreases the cell potential. As acid is added to water, a certain amount of hydronium will be present in a certain volume of solution.
Question what happens when the concentration of solutes decrease in the guard cells? Upon mixing 3 m of n204 and 2 m of no, the. Therefore increasing the solutions concentration will certainly increase absorbance.
However, if the zn2+ ions aren't involved in. A water potential decreases b water potential increases c osmotic potential increases d none. Molar conductivity with concentration molar conductivity of a solution at a given.
2) as the ka increases for a given acid, what will happen to the pka? According to beer’s law, a = εlc, a substance’s concentration and absorbance are directly proportional under ideal conditions: Now when two solutions are mixed the volume the mixture is increased and so the same amount of.
As we know boiling point ∝ 1 vapor pressure, so. If the ph of a solution is 10 what is the poh? 1) what is the h+ concentration in a solution that has a poh = 11?
For instance, if your lemonade was too tart, you would add more water to decrease the concentration. Solution verified by toppr correct option is a) the concentration of hydronium ions decreases when an acid is diluted because on adding water the h+ ions of the acid and hydroxyl ions of. In an aqueous solution, decreasing the concentration of hydrogen ions will increase the concentration of hydroxide ions in proportion because their concentrations when multiplied.

Solutions Ppt Video Online Download
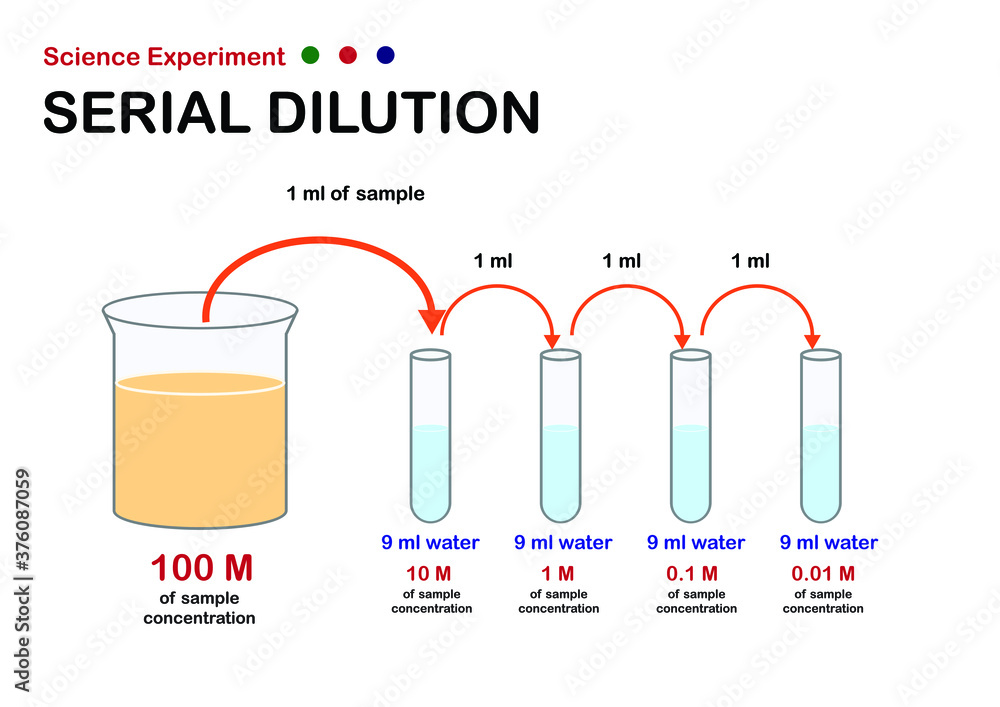
Scientific Experiment Diagram Show Concept Of Serial Dilution For Decrease Concentration Of Sample Solution Stock Vector | Adobe Stock
