Can you tell how many valence electrons are in transition metals? Transition metal, any of various chemical elements that have valence electrons—i.e., electrons that can participate in the formation of chemical bonds—in two shells instead of only one.
Valence Electrons (Video) | Khan Academy
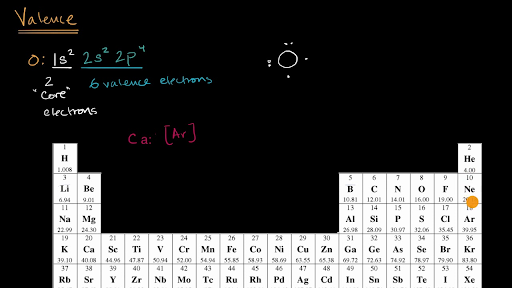
The number of valence electrons.
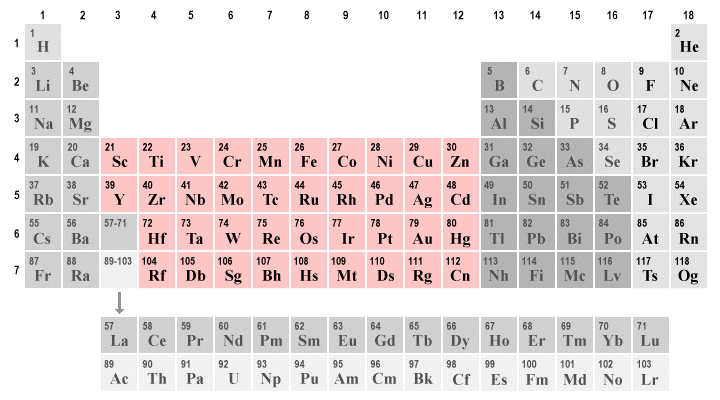
Transition metals valence electrons. A valence electron can either absorb or release energy in the form of a photon. Perhaps this is where you are getting the idea of two valence electrons. Occasionally, electron counts can be as low as eight electrons.
The period 6 and 7 transition metals also add core ( n − 2) f14 electrons, which are omitted from the tables below. These cases often occur in early transition metals, such as titanium or tantalum. The transition metals have at least two valence electrons, and more.
These metals have a long way to go to get to eighteen electrons, and sometimes they cannot fit that many ligands in. Transition metals has ____ valence electrons 1 Transition metals possess several interesting properties such as they can form ions with different charges.
Valence electrons also determine the electrical conductivity of an element. Some characteristic transition metal ions in turn can form brightly colored solutions. Click here 👆 to get an answer to your question ️ 15.
This tendency is called the octet rule, because each bonded atom has 8 valence electrons including shared electrons. Transition metals has ____ valence electrons johnylit johnylit 01/09/2021 chemistry college answered 15. Is this because the d orbital tends to fill either halfway or completely full?
Most have 2 valence electrons because of the p orbitals in the shell of their period number fill up before the d orbitals. Most transition metals have an electronic configuration that is ns2 (n−1)d, so those ns2 electrons are. Predict the electron configuration of the fe 3+ ion.
Below is a table of the oxidation states that the transition metals can or cannot form. Similarly, a transition metal tends to react to form a d 10 s 2 p 6 electron configuration. An atom consisting of a closed shell of valence electrons will usually be chemically inert.
But for most of the transition and inner transition elements, the valence electrons are the electrons present in the shells outside the noble gas core. Group 2 (ii) (alkaline earth metals) 2: Most transition metals have 2 valence electron valence electron are the sum total of all the electrons in the highest energy level (principal quantum number n).
A valence electron can exist in the inner shell of a transition metal. Group 1 (i) (alkali metals) 1: Click here to check your answer to practice problem 1
Scandium (sc), titanium (ti), vanadium (v), chromium (cr), manganese (mn), iron (fe), cobalt (co), nickel (ni), copper (cu), and zinc (zn). No logic here, only tradition. Periodic table group valence electrons;
In the first row of the transition metals, the ten elements that can be found are:

Valence Electrons: Check Electron Dot Diagrams - Embibe

Electronic Configuration - How Many Valence Electrons Do Elements In The D Block Have? - Chemistry Stack Exchange