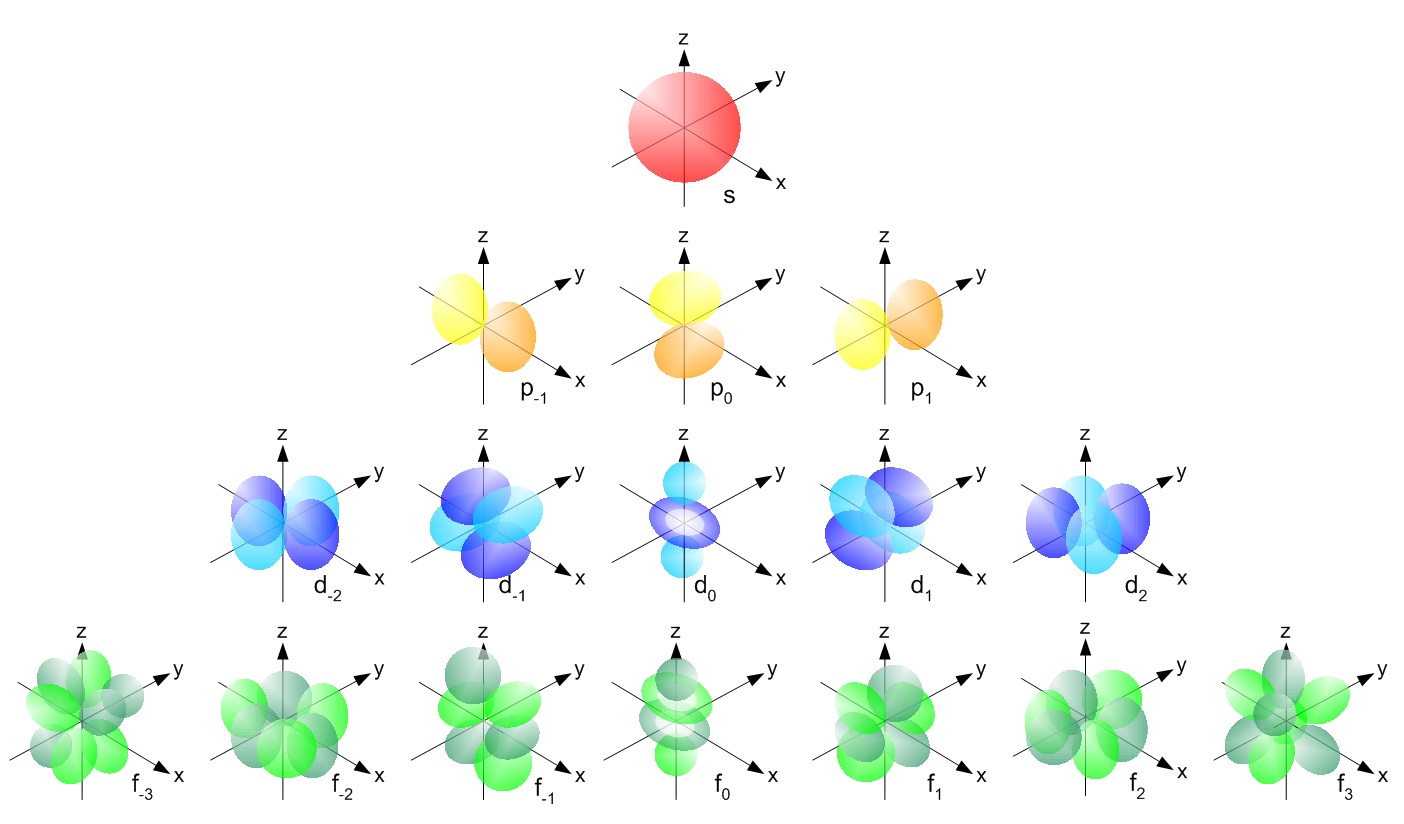
The orbitals grow bigger as the energy levels increase and. 1 s s s and 2 s s s orbitals both have the same spherical shapes.
How Do You Draw S,P,D,F Orbitals? | Socratic
The planes alignment happens every ~7.5.
Sketch of a s orbital. And s orbital, for example, is spherical in shape, which is centered around the. The sketch is about 800 pm wide, the coordinate (x,y,z) axis are all shown. Sketch of a polar view of the swarm orbital planes:
How would the 4d orbitals differ from the 3d orbitals? Each box will hold a. Polar view sketch of swarm’s orbital planes.
Here is a sketch of a 2py orbital. A p orbital consists of two lobes of electron density on either side of the nucleus. See the answer see the answer see the answer done loading.
Since 2 s s s electrons are farther from the nucleus, this means 2 s s s orbital is a larger orbital and farther from the. An illustration of the shape of the 3d orbitals click the images to see the various 3d orbitals there are a total of five d orbitals and each orbital can hold two electrons. An s orbital is a sphere.
The idea often orbital, was introduced. Around the atomic nucleus, the s orbitals are found to be spherically symmetric like a very hollow ball with a nucleus at the center. This problem has been solved!
In contrast to his concept of a simple circular orbit with a fixed radius, orbitals are mathematically derived regions of space. These orbitals contain a number of boxes that can hold a number of electrons. Arbiters can have the same shape, but different orientations.
The plot of angular wave functions or square of angular wave functions (probability functions) give us the shapes of orbitals.these two plots differ only slightly. 1s here are some boxes for you to practice drawing s. Let us consider the individual.
And if you are drawing his by hand, the loop does not have to be an exact circle. P orbital when n = 2, two sublevels are possible: The fifth d orbital is shaped like an elongated.
Suppose an atom with its nucleus at the origin has an electron in a 2py orbital. In two dimensions, we draw it as a circle. An orbital is the quantum mechanical refinement of bohr’s orbit.
It is also possible to show the orbital as a simple loop.
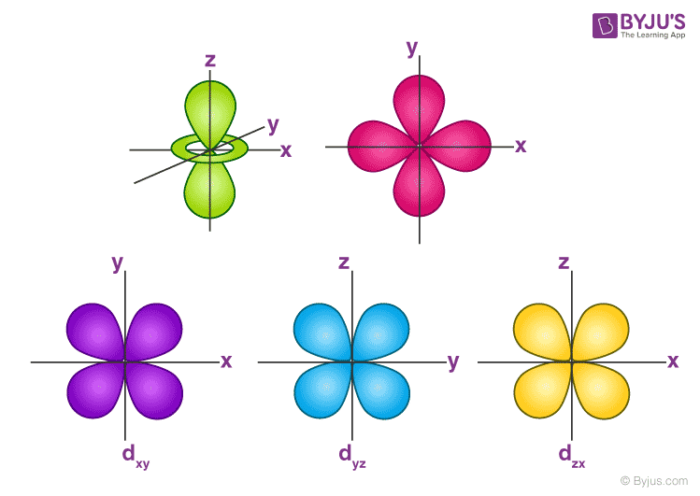
Orbitals Chemistry (Shapes Of Atomic Orbitals) - Shape Of S, P, D, And F Orbital