If unpaired electrons are present in an ion/molecule, then it is. The energy diagram of o2molecule is:

File:valence Orbitals Of Oxygen Atom And Dioxygen Molecule (Diagram).Svg - Wikipedia
O2 is paramagnetic as it has unpaired electrons.
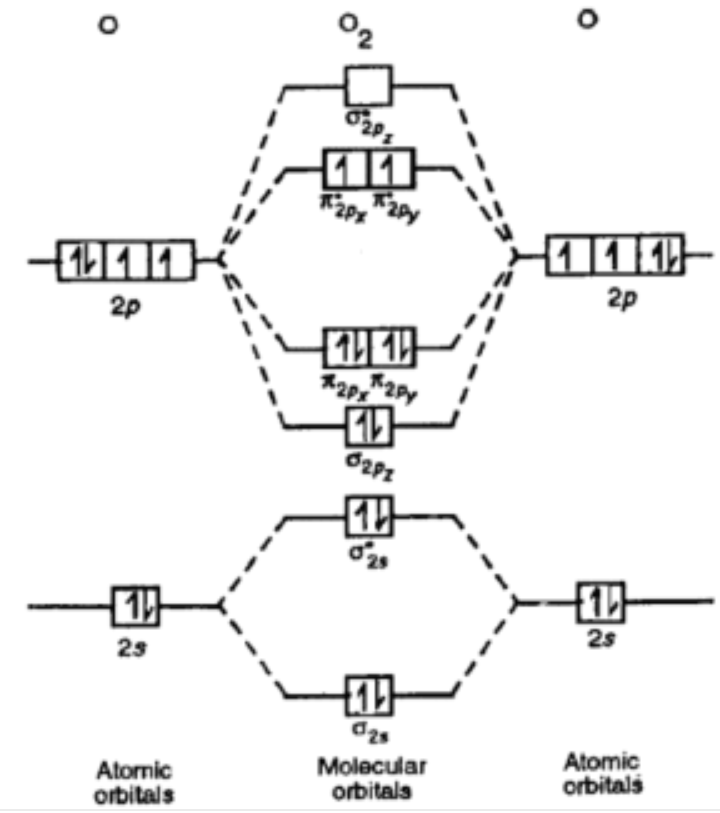
O2 paramagnetic or diamagnetic. The electron would be removed from the π orbital, as this is the highest in energy. H2 ( hydrogen ) is a diamagnetic. And that shows o2 with it's 16 electrons is paramagnetic because of the 2 half filled bonding orbitals and it shows.
O2,o2− and o22− are paramagnetic species. O₂ is paramagnetic because there are more unpaired electrons and n₂ is diamagnetic because there are no unpaired electrons. Molecule is paramagnetic in nature.
Oxygen is paramagnetic mainly because it consists of two unpaired electrons in its last molecular orbital. Would it be paramagnetic or. We know that one oxygen atom has 8 electrons hence, a molecule will have 16 electrons.
Paramagnetic (has an odd number of electrons, so at least one must be unpaired). Paramagnetism is a form of magnetism whereby certain materials are weakly. O+ 2 has 1 fewer electron than o2 which is what gives it the positive charge.
The neutral oxygen is paramagnetic according to mo theory because it ends up with two unpaired electrons in two degenerate pi antibonding molecular orbitals. Yes o2 (2+) is diamagnetic. Since, all the orbitals have paired electrons.
In o2 state, the 2p1x and 2p1y orbitals have one. Li2 has all of its electrons neatly paired up in the orbitals. What is paramagnetic and diamagnetic ?
Is determined by molecular orbital theory. To form o 2 2 − , one extra electron enters the 2 p x orbital and one more electron enters the 2 p y orbital. This problem has been solved!
Correct option is a) the given statement is true. This can be proven if we look at the molecular orbital diagram of oxygen. O 2 and o 2− are paramagnetic while o 3 and o 22− are diamagnetic.
Is o2 diamagnetic or paramagnetic? The molecular orbital diagram is shown below. This molecule will show diamagnetic.
The electrons in π∗2px and π∗2py remain unpaired. A strong electromagnet is placed next to the sample, which is on a balance. Peroxide ion and hydrogen peroxide:
Why is o2 2 paramagnetic or diamagnetic? A molecular structure that results in unpaired electrons in degenerate mos, like o2, is called paramagnetic. We can work this out by looking at the molecular orbital diagram of o2 o2 (2+) has two fewer electrons than o2 which is what it gives it positive.
The number of unpaired electrons in.

