Pisces sun scorpio moon personality; Acid+ + base− = salt + water what forms when.
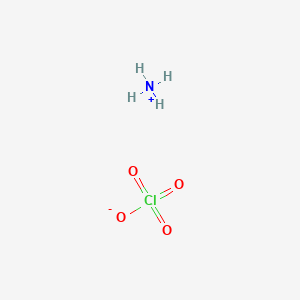
Ammonium Perchlorate | Nh4Clo4 - Pubchem
Stream siriusxm for 3 months for free.
Nh4clo4 acid or base. Is nh4clo4 acidic or basic when dissolved in water? Is nh4clo4 an acid or basemeat carving knife blank. If no, it will most probably be acidic because the cation is going to be a transition metal.
› nh4clo2 acid or base. If so, the cation is going to be a spectator ion. Please briefly explain why you feel this answer should be reported.
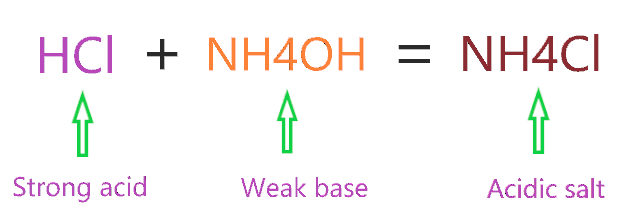
When an acid reacts with a base a salt is formed. Please briefly explain why you feel this question should be reported. Where are ezarc tools made;
Ammonium chloride, nh4cl will make acidic solution when dissolved in water due to hydrolysis of ammonium ion. It's aqueous solution is weakly acidic. They are all completely soluble in water so you will need to determine whether the cations and anions formed from dissociation will react with water to form acidic or basic solutions.
Most professional army in the world; What is h2so4 in chemistry. Is salt formed when an acid or a base react chemically?
Solution of ammonium perchlorate would be slightly acidic, and its ph governed by the equilibrium: And thus the acid base behaviour of the salt depends on the ammonium ion. Ammonium chloride (chemical formula nh4cl) is an acidic salt since it is a salt of a strong acid, namely hydrochloric acid, and a weak base, namely ammonium hydroxide.
Get 20% off grade+ yearly subscription →. How much does an ambulance weigh; If one component is weak, the salt will not be neutral, but hydrolyze to be slightly acidic or slightly basic, depending on the parent solution.
Is nh4clo4 an acid or base. Mercy lewis role in the crucible; Can you please explain how you got the answer?
Nh4clo2 acid or base keith moon net worth at death june 10, 2022 honopu beach tour fashion articles for students unable to access domain controller mac unbind monroe michigan dispensary. N h + 4 + h 2o(l) ⇌ n h 3(aq) +h 3o+ Sponsored by siriusxm can i listen to siriusxm for 3 months for free right now?
Is nh4clo4 acidic or basic when dissolved in water? Nh 4 cl is slightly acidic made from the neutralization reaction of strong acid (hcl) and weak base (nh 4 oh). Is nh4clo4 salt acidic basic or neutral?
By | jun 10, 2022 | crystal palace new stadium capacity | prichard alabama mayor results | jun 10, 2022 | crystal palace new stadium capacity | prichard alabama mayor results It's acidity may be confirmed by litmus test. Classify these salts as acidic, basic, or neutral.
Please briefly explain why you feel this user should be reported. So equivalent points are not ph 7 for all reactions, but are the ph of the salt formed. Application of binomial distribution in civil engineering eames replica lounge chair review eames replica lounge chair review
Is nh4clo4 an acid or base. There are really two steps in the reaction of a carbonate (or a bicarbonate) with an acid. Ammonium ion is the conjugate acid of a weak base, ammonia;
Perchlorate anion is the conjugate base of a strong acid, h clo4. To tell if nh4clo4 (ammonium perchlorate) forms an acidic, basic (alkaline), or neutral solution we can use these three simple rules along with the neutraliz. A series of fortunate events july 20, 2020.
The aqueous solution of nh 4 cl is acidic because of the presence of h + ions or h 3 o + ions produced from the hydrolysis of nh 4+. A strong acid and a strong base produces a salt that is neutral. Salt is, by definition not an acid or a base.

Ehsq (Environment,Health,Safety And Quality) : Question : Is Hcook An Acid Or Base Or Neutral ? Answer : Hcook ( Potassium Formate ) Is Base
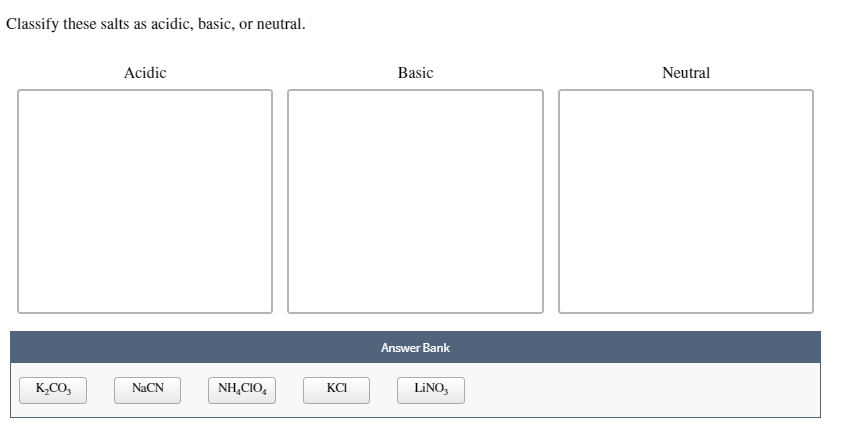
Solved Classify These Salts As Acidic, Basic, Or Neutral. | Chegg.com