Ml is the magnetic quantum number and defines a specific orbital. If we putθ =0∘or180∘, we’ll get the condition for parallelism
Solved 11. What Set Of Quantum Numbers Is Allowed? N= =2, | Chegg.com
I.e.cos (θ) = (l1.l2+m1.m2+n1.n2) where l1,m1,n1 are the direction cosines of one line and l2,m2,n2 are the direction cosines of another.
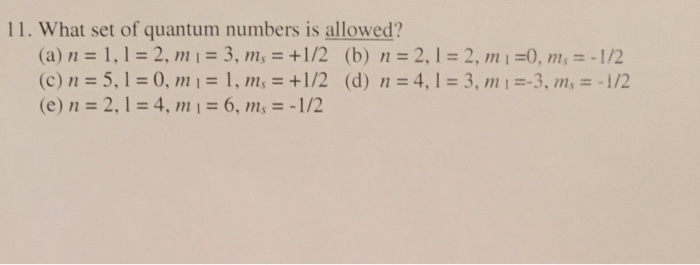
N=2 l=1 m1=-1. The 2p1 electron, then they would be: The magnetic quantum number, ml, tells you the exact orbital in which the electron is located. So if it was n=5, l=2, ml=2 it would still be 2 right?
Therefore, let l, m, n be the direction cosines of the line which is perpendicular to the line with direction cosines l1, m1, n1 and l2, m2, n2, substituting the values from equations (5) and (6) in equation (4), we obtain Best answer it is given that l1, m1, n1 and l2, m2, n2 are the direction cosines of two mutually perpendicular lines. Post by 304310808 » sun dec 08, 2013 4:55 am.
Question the set of quantum numbers if any for, n=2, l=2, m 1=0: See the answer see the answer see the answer done loading Answer to solved n = 4, l = 4, m_1 = +3 n = 4, l = 4, m_1 = +4 n = this problem has been solved!
Answer to solved which of these are allowed? If l1, m1, n1, l2, m2, n2 and l3, m3, n3 are the direction cosines of three mutually perpendicular lines,then prove that the line whose direction cosines are proportional to l 1+ l 2+ l 3, m 1+ m 2+ m 3 and n 1+ n 2+ n 3 makes equal angles with them. It is in the second block of 2p subshell (m=0, l=1 gives possibility of.
The ml just tells you what orbital it is! Class 10 science ncert solutions. 1 s 2 2 s 2 2 p 2 so we know the final electron is in second (n=2) orbital.
Ncert dc pandey sunil batra hc verma pradeep errorless. L is the angular momentum quantum number and defines the orbital type. N = 3, l = 2, ml = 1, ms
Ms is the electron spin and is either spin up or spin down. The quantum numbers n, l, ml, and ms are used to identify a specific electron in an atom. Ncert easy reading alleen test solutions blog about us career
In this lecture i discussed how to construct dfa for following infinite language.σ=a,bl23.1 =a^nb^m l23.2 = n,m ≥0𝛴=𝑎,𝑏,𝑐l23.3 ={. If you are referring to the last electron, i.e. Class 12 class 11 class 10 class 9 class 8 class 7 class 6.
It is in the p (from l=1) sub shell. Assuming that n=2,l=1,m=0,and s=+1/2 is giving the last electron we are able to find the number of total electrons. If the direction cosines of two lines are (l(1), m(1), n(1)) and (l(2), m(2), n(2)) and the angle between them is theta then l(1)^(2)+m(1)^(2)+n(1)^(2)=1= books.
Fri sep 20, 2013 10:00 am. Two lines being parallel to each other means the angle between these lines is0∘we know how to calculate the angle between two lines. And remember each orbital hold 2.
Class 9 science ncert solutions. A describes an electron in a 2s orbital b describes one of the five orbitals of a similar type c describes an electron in a 2p orbital d is not allowed medium solution verified by toppr correct option is d) we have, forspdf l=0123 nown=2l=2⇒d but 2d\quad is not possible. N is the principal quantum number and defines the energy level.
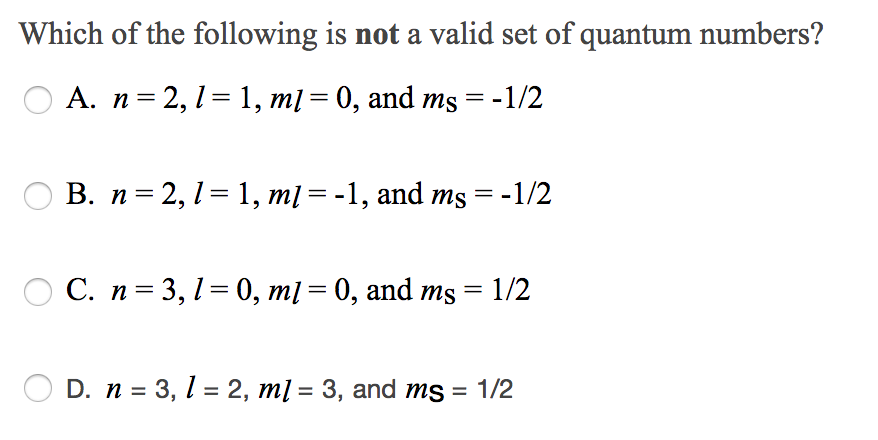
Solved Which Of The Following Is Not A Valid Set Of Quantum | Chegg.com
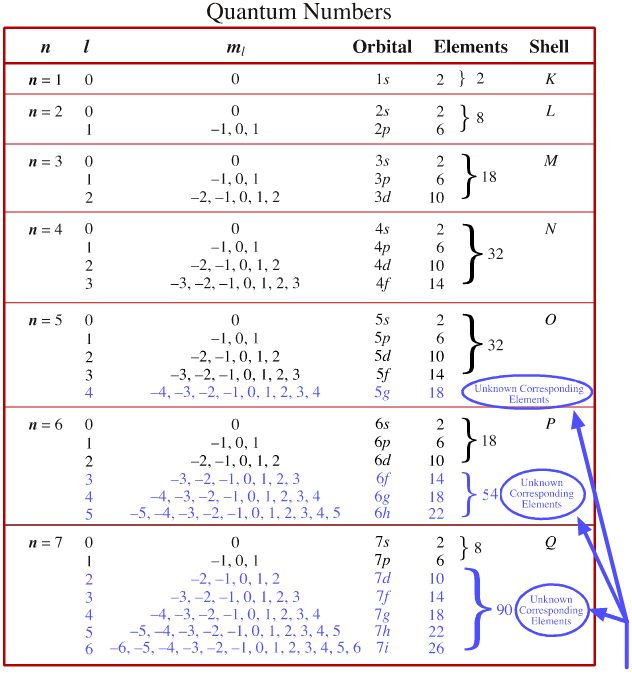
How Many Electrons Can Have N = 3, L = 2, M_L = 2, M_S = -1/2? | Socratic