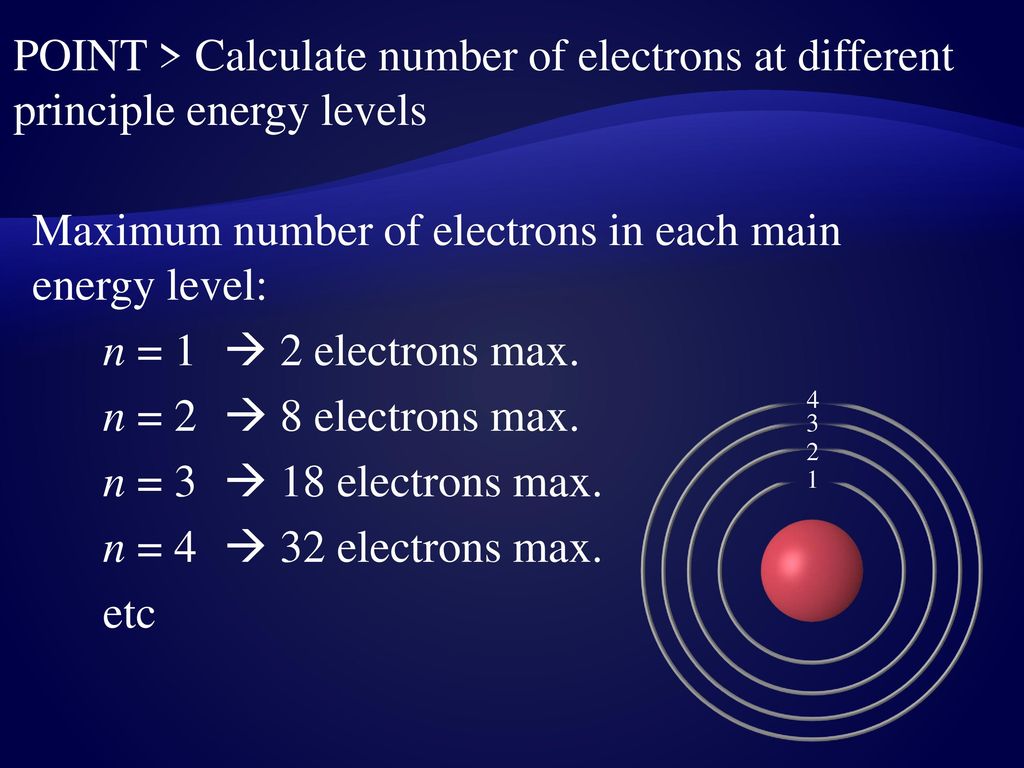
N shell, n = 4. 3rd shell can hold 18 electrons.
4.2B Quantum Numbers And Atomic Orbitals - Ppt Download
The s sublevel has one orbital (with a maximum of 2 electrons) and for the p sublevel, we know that p has 3 orbitals and a maximum of 2 electrons in each orbital.

Max number of electrons in n=4. Join / login >> class 11. This means that the fourth energy shell can hold a. The maximum number of electrons that can have those two values for n and ml is 4.
Simple answer would be to say that nitrogen bonds with any element with only 4 orbitals,1s and orbital and 3p orbital.so its covalency is limited to 4. Therefore, a maximum of no. Of electrons = 2 ⋅ 4 = 8 electrons.
It covers about 8 cases. Solution for what is the max. Since each orbital can hold a maximum of two electrons the number of electrons that can share the two quantum number n=3 and ml=−2 will be equal to 2 each having.
2 × 4² = 32. This chemistry video tutorial explains how to determine the maximum number of electrons given a set of quantum numbers such as n, l, ml, and ms.my website: 32 electrons here n is the principal quantum number that describes the energy shell.
Advertisement advertisement hannahrenee98 hannahrenee98 there will be 32 electrons in the n=4 shell. This video shows you how to determine or calculate the maximum number of electrons using allowed quantum numbers (n, l, ml, and ms). N = 5 s = + 1/2.
Half of these electrons are spin up (ms=+1/2) and. Thus, 1st shell can hold 2 electrons. Of orbitals = n2 = 22 = 4 orbitals.
In the shell n=4, we can have 32 electrons. What is the maximum number of electrons in n 4? 2nd shell can hold 8 electrons.
Maximum number of electron that may be present on 4forbital is a 2 b 4 c 7 d 14. To determine the maximum number of electrons present in the 4f orbital, first we need to find out. Of electrons = 2n2 in this case, the second energy level holds a total of no.
2+8+18+32=60 electrons we need to keep in mind that the 2 n 2 rule tells us how many electrons can be in the shell with the principal quantum. The energies e 1 and e 2 of two radiations are 25 ev. L=4 refers to the g orbital which has 18 electrons if you add up all the electrons in the shell n=5, you will get a total of 50 electrons.
Of electrons in the given set of quantum numbers? 4th shell can hold 32 electrons. Maximum number of electrons in a subshell with l =3 and n=4 is 14 16 10 10 798 views switch flag bookmark 84.
Hence, the number of electrons in n = 4 energy level will be 32. 2 × 3² = 18. However, i was previously taught that the maximum number of electrons in the first orbital is 2, 8 in the second orbital, 8 in the third shell, 18 in the fourth orbital, 18 in the fifth.
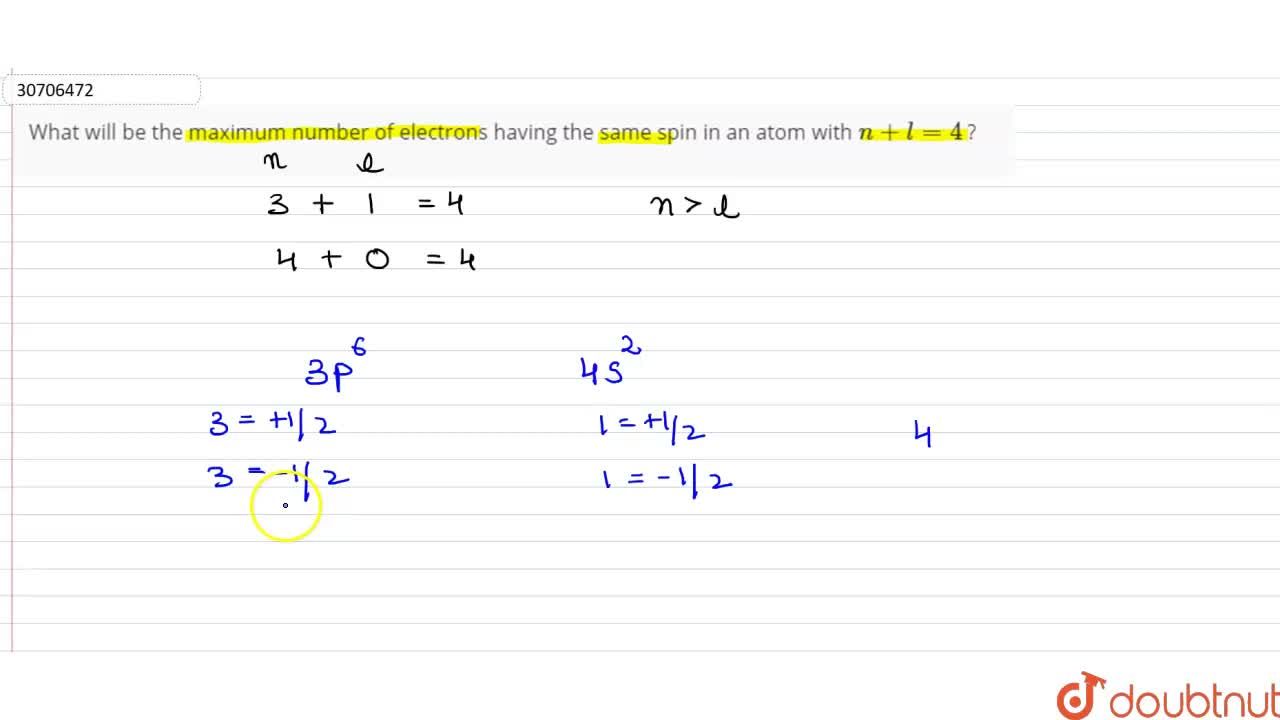
What Will Be The Maximum Number Of Electrons Having The Same Spin In An Atom With N +L=4 ?

How To Determine The Maximum Number Of Electrons Using Allowed Quantum Numbers - 8 Cases - Youtube