Since 1s can only hold two electrons the. 119 rows the period or row numbers 1 through 7 are the energy levels of the elements.

Electron Configuration For Lithium (Li)
To write the configuration for the manganese ions, first we need to write the electron configuration for just manganese (mn).

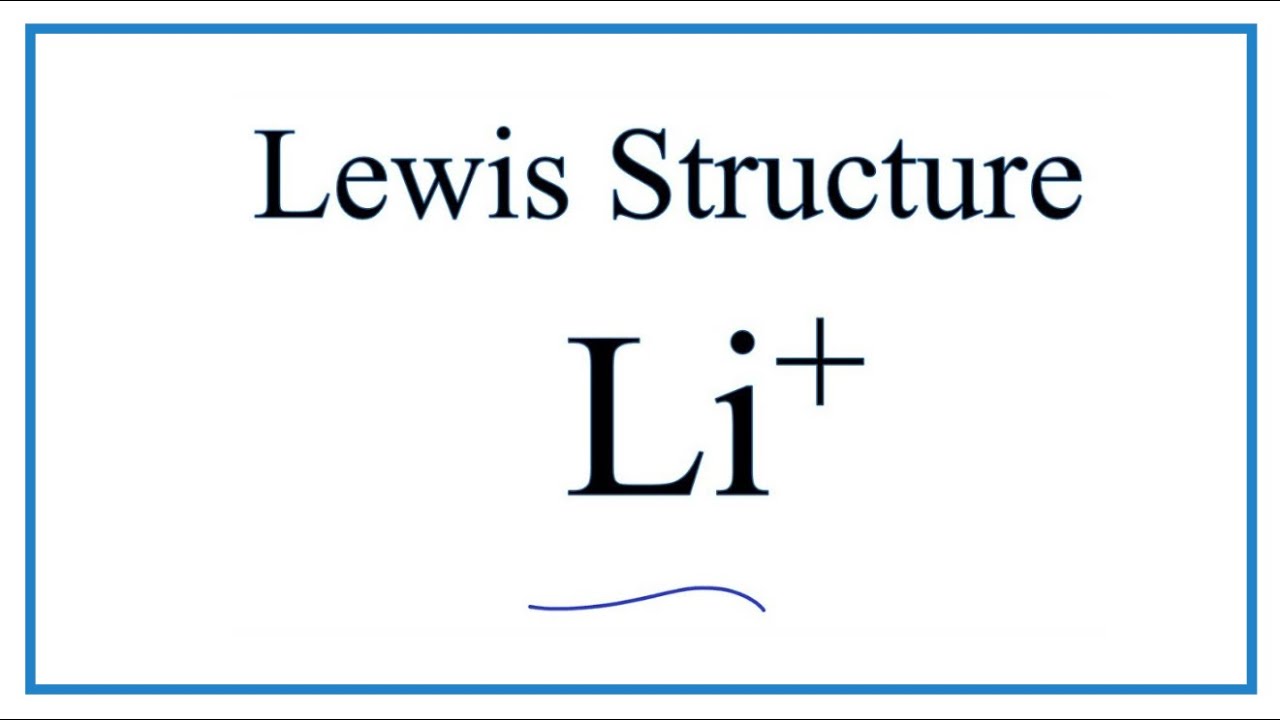
Li+ electron configuration. In writing the electron configuration for lithium the first two electrons will go in the 1s orbital. The elements that form bonds by donating electrons are called cation. The li stands for lithium which is the third in the table of elements this means its electronic configuration will be 2:3.
Part a what is the electron configuration of li+? The s orbital holds a maximum of 2 electrons. Lithium atom donates an electron of the last shell to turn into a lithium ion (li + ).
We first need to find the numb. Use the inner electron configuration format. Lithium is the third element with a total of 3 electrons.
Solution for using electron configurations, show how li+ and k+ are formed. What is the electron configuration for a lithium ion? (a) compare the electron configurations and atomic radii of rubidium and silver (see figure 7.6 above or in your text).
What is the full electron configuration of li? This electron configuration shows that the nickel atom has two unpaired electrons. The li stands for lithium which is the third in the table of elements this means its electronic configuration will be 2:3.
Li+ electron configuration (lithium ion) wayne breslyn 556k subscribers 202 dislike share 33,404 views jun 17, 2019 in this video we will write the electron configuration for. The p orbital can hold 6. Lithium leaves an electron and turns.
So the valency of nickel is 2. When a nickel atom is excited, then the nickel atom absorbs energy.
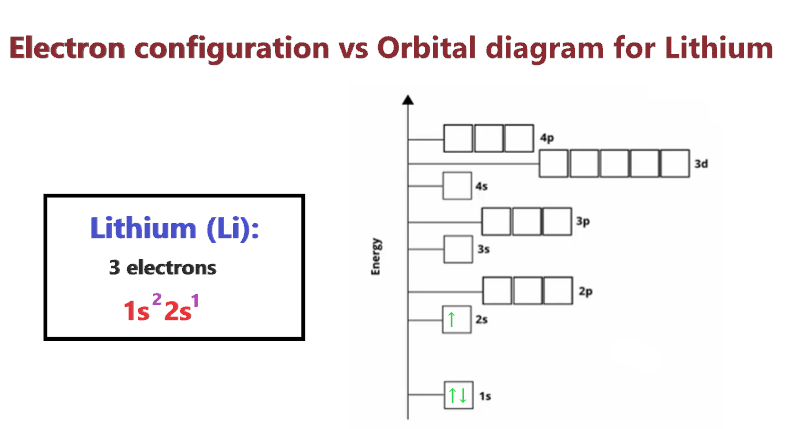
Lithium Orbital Diagram, Electron Configuration, And Valence Electrons