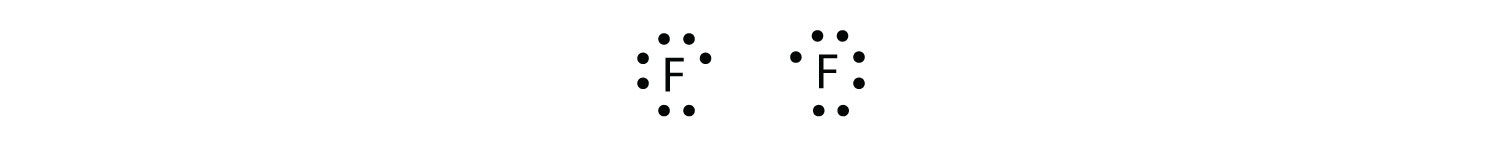Is tio2 a ionic or covalent bond? Find an answer to your question is ff non polar covalent bond?
A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms.these electron pairs are known as shared pairs or bonding pairs, and the stable balance of attractive and repulsive forces between atoms, when they share electrons, is known as covalent bonding.
Is ff covalent. 1 see answer advertisement advertisement adityakaushik4060 is waiting for your help. The shorter the bond length, the more efficient is the overlapping of atomic orbitals, leading to stronger covalent bond. Consider the hydrogen chloride (hcl) molecule.
Mixed bond (not ionic, not covalent). The order of bundles can affect. A bromine molecule is called a diatomic molecule since it has only two atoms.
The ionic bond is formed between the two atoms when their electronegativity difference occurs more than 1.7. These elastomers exhibit good thermal stability and excellent hydrophobic property and can be applied as adhesives for bonding iron sheets to hold the weight of 500 g. For many molecules, the sharing of electrons allows each atom to attain the.
F atom has a smaller atomic radius than cl atom, because f atom has one less filled electron shell. Wang, xiaoyi xu, +4 authors hongzheng chen materials science, chemistry A polar covalent bond exists when atoms with different electronegativities share electrons in a covalent bond.
The covalent bond is formed between the two atoms when their electronegativity difference occurs less than 1.7. Chlorine has a higher electronegativity than hydrogen, but the chlorine atom’s attraction for electrons is not sufficient to remove an. Complete step by step answer:
Adityakaushik4060 adityakaushik4060 27.08.2021 chemistry secondary school answered is ff non polar covalent bond? Bromine will normally form one covalent bond. When two atoms bond together, they form a molecule.
Covalent organic frameworks (cofs) have been utilized as molecular sieves to adsorb or remove or separate a wide range of substances. Hydrogen fluoride is a covalently bonded polar molecule because of the unequal sharing of electrons among more electronegative fluorine and the less electronegative hydrogen atom. Mfsy was able to target residues that were elusive for previous unnatural amino acids, and permitted engineering of various proteins including affibody, nanobody, and fab into covalent binders that irreversibly.
For example, the h and f atoms in hf have an electronegativity difference of 1.9, and the n and h atoms in nh3 a difference of 0.9, yet both of these compounds form bonds that are considered polar covalent. Structural design, synthesis, and application s. Each atom in hcl requires one more electron to form an inert gas electron configuration.
Does helium want to bond? (employees and sales figures are modelled). The degree of ionicity is about 70%, 30% covalent character.
What is the ionic/covalent of ff: Well either it 'npm i' didnt finish correctly or you there is something wrong with the way covalent core is being imported sandeep kalra. What kind of bond is ff?
Covalente has 2 total employees across all of its locations and generates $372,000 in sales (usd). The forces of attraction/repulsion between two atoms (when they share a bond pair/ electron pair) are known as a covalent bond. I am doing a 'fresh' npm i at the same time, this is something i found on net angular/angular#10618.
They are also called a molecular bond. Covalente is located in pithiviers, centre val de loire, france and is part of the other investment pools and funds industry. The “covalence” word was termed by the scientist named irving langmuir, which states about the number of electron pairs shared by the neighbouring atoms.
Covalent Vs Charge-Shift Nature Of The Metal–Metal Bond In Transition Metal Complexes: A Unified Understanding | Journal Of The American Chemical Society