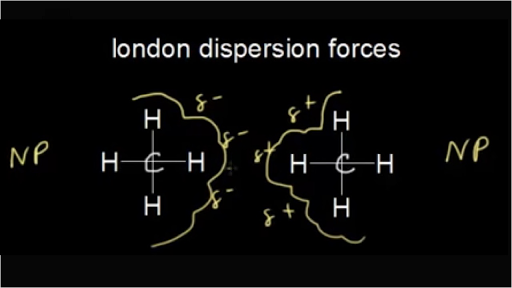
At room temperature, ccl4 is a liquid while ch4 is a gas. The intermolecular forces that exist between molecules of ch4 are called dispersion forces.
Intermolecular Forces (Video) | Khan Academy
We draw this covalent bonding as a lewis structure (see diagram).

Intermolecular force ch4. What type of intermolecular forces are present in ch 4? The lines or sticks as we say represent the covalent bonds. The total number of electrons present in a ccl4 molecule is 32, while that in a ch4 molecule is 8.
In ch4(methane), the only intermolecular forcesare london dispersion forces. Methane ch4 is a covalent compound with exactly 5 atoms that are linked by covalent bonds. Methane ch4 is a covalent compound with exactly 5 atoms that are linked by covalent bonds.
Therefore the strongest intermolecular forces between ch4 molecules are van der waals forces. There are four bonds from a central carbon (c) linking or bonding it to four hydrogen atoms (h). In a single molecule of ch4 you would have intramolecular forces that are covalent bonds.
Which has stronger intermolecular force: What intermolecular force is ch4 if you are seeing this message, it means that we are having trouble uploading external resources to our website. See answer (1) best answer.
What is the strongest intermolecular force in ch4? The lines or sticks as we say represent the covalent bonds. The intermolecular forces that exist between molecules of ch4 are called.
If you are behind a web filter, make sure that the *.kastatic.org and *.kasandbox.org domains are unlocked. What are the inermolecular forces present in ch4? Intermolecular forces for ch4 (methane) 2,509 views jan 12, 2022 wayne breslyn 561k subscribers 38 dislike share in this video we’ll identify the intermolecular forces for ch4 (methane).
We draw this covalent bonding as a lewis structure (see diagram). Which is an intermolecular force quizlet? London dispersion forces are the result of temporary dipoles in molecules that are created when electrons are.
Methane ch4 is a covalent compound with exactly 5 atoms that are linked by covalent bonds. The cental atom in each of these molecules is c, n and o respectfully, of these n and o are There are four bonds from a central carbon (c) linking or bonding it to four hydrogen atoms (h).
The lines or sticks as we say represent the covalent bonds. In a single molecule of ch4 you would have intramolecular forces that are covalent bonds. We draw this covalent bonding as a lewis structure (see diagram).
The only intermolecular forces in methane are london dispersion forces. There are four bonds from a central carbon (c) linking or bonding it to four hydrogen atoms (h). Ccl4 has a much higher boiling point than that methane.

Intermolecular Forces For Ch4 (Methane) - Youtube

Intermolecular Forces - Hydrogen Bonding, Dipole-Dipole, Ion-Dipole, London Dispersion Interactions - Youtube