The ph to h+ formula that represents this relation is: [a−] = [h 3o+] since you know the molarity of the acid, ka will be ka = [h 3o+]2 [h a] answer link
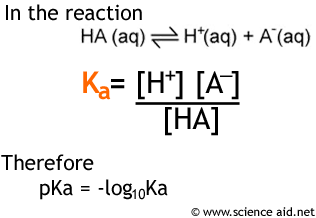
Acids And Bases: Ph, Kw, Weak Acids And Bases, And Buffers - Scienceaid
They describe the degree of ionization of an acid or base and are true indicators of acid or base strength because adding water to a solution will not change the equilibrium constant.

How to calculate the ka without ph. Answer of calculate the ph of a 0.100 m sodium formate (nacho2) solution. To calculate ph all you need is the h+ ion concentration and a basic calculator, because it is a very straightforward calculation. Nacho2 (aq) + h2o (l) ?
How to calculate the ka or kb of a substance without given the ph expert solution. Then, we use the ice table to find the concentration of the products. This video shows you my shortcut for skipping the ice chart and skipping the quadratic equation for weak acid calculations.
Ka and pka relate to acids, while kb. Answer of calculate the ph of a 0.100 m sodium formate (nacho2) solution. Solution for how to calculate the ka or kb of a substance without given the ph.
Because an acid dissociates primarily into its ions, a high ka value implies a powerful acid. To calculate ka, we divide the concentration of the products by the concentration of the reactants. The ratio b/a can also be expressed as the fraction of the total concentration present as protonized acid (ya):
Ka and ph calculations for weak acids can be trick on the mcat if you attempt a general chemistry approach. A ph less than 7 indicates an acid, and a ph greater than 7. I encounter such question in destroyer.
This video shows how you can calculate the ka of an acid, if you're given the ph of the solution (and its concentration, of course). Solutions with a ph that is equal to 7 are neutral. How to calculate the ka or kb of a substance without given the ph.
Ka = [h 3o+] ⋅ [a−] [h a] if you have a 1:1 mole ratio between the acid and the hydronium ions, and between the hydronium ions and the conjugate base, a−, then the concentration of the latter will be equal to that of the hydronium ions. Ice tables and quadratic equations are not only a waste of time, but nearly impossible without a calculator. If the ph is higher, the solution is basic (also referred to as alkaline).
Password must contain 6 to 25 characters without space. Formula and vocabulary used in calculating the ka of a weak acid from ph ph: 1 vote and 16 comments so far on reddit
Ka, pka, kb, and pkb are most helpful when predicting whether a species will donate or accept protons at a specific ph value. It requires a modest understanding of the properties of l. Hasselbalch equation to calculate ph ph = pka + log ( mol salt/ mol acid) ph = 4.76 + log 0.001/0.0015) ph = 4.76 + log 0.666 ph = 4.76 + ( * 0.18) p continue reading rakesh kumar meher bsc in chemistry, gangadhar meher university, sambalpur (graduated 2017) 4 y related is a blood buffer solution?
Ka=(ha) where & are molar concentrations of hydronium ion conjugate base and weak acid at equilibrium.ka=(ha) where & are molar concentrations of This chemistry video tutorial explains how to calculate the ph of a solution without a calculator. A measure of hydronium ion concentration in a solution.
You just need to know the equilibrium concentration of the acid and its conjugate base. Calculate the ka of 2m hypochlorus acid (hcio) if its ph is 5. To find ka, you will need to use the ice (initial, change, equilibrium) table and the following formula.
This can be simplified (assuming ka is quite small) to ka= [concentration of h3o]^2 / [concentration of conjugate acid] A high ka value indicates that the reaction arrow promotes production formation.

Find The Ka Of An Acid (Given Ph) (0.1 M Hypochlorous Acid) Example - Youtube

How To Calculate Ka Of An Acid From Ph ? (With An Example) - Youtube