O2 has total of 12 valence electrons. Four electrons are gained by one oxygen molecule.

O2 2- (Peroxide) Ion Lewis Structure
The conditional charge of an atom’s atom is called the oxidation state.
How many electrons does o2 have. Electronic configuration of o 2 is he]2s 22p 4. In the textbook, it says that the molecule o2 has 2 unpaired electrons. How many electrons are there for the o 2 or o2 ion.
Oxygen has three stable isotopes, 99.76%^16o, 0.04%^17o, and 0.20%^18o. A neutral oxygen atom as also has 8 electrons. It can be either positive or negative.
However, when i draw the lewis structure (and count the number of valence electrons, which happen to be even), no electrons are unpaired. In this case, the oxygen atom carries a negative charge. The terms “ oxidation degree ” and “ valence ” may not be the same, but they are numerically almost identical.
Each o has 6 valence electrons, but when they bond they donate electrons. How many electrons are gained in the half reaction o2 plus electrons 2 o2? Equal electronegativity means that there are no partial charges for each element.
Because the 1s orbital can only store two electrons, the next two electrons for oxygen are placed in the 2s orbital. Medium view solution > view more How many electrons does o2 negative have.
Valence refers to the ability of an atom form bonds. The initial two electrons in the electron configuration for oxygen will be in the 1s orbital. If one o donates 1 electron, the other o donates 1 electron.
From the molecular orbital diagram it is clear that o 2 has 2 unpaired electron. Oxygen has the atomic number 8, which means the nuclei of its atoms have 8 protons. This means that it has gained two electrons from another element, such as.
Get the answers you need, now! How many electrons does oxygen have? Since they both have the same exact electronegativities it is likely they will each donate the same number of electrons as the other.
And clearly 2+4=6 valence electrons are there in outermost shell. Medium view solution > which mo is the homo of o 2 molecule? There are two additional electrons in o2, making the total number of valence electrons in o2 eight.
O2 is a nonpolar molecule. This means that an oxygen atom contains eight electrons. This is because its electronic configuration is :
O2 has 6 valence electrons. 0 0 similar questions how many bonding electrons are there in 2p mo of o 2 molecule? Ryan trujillo studied computer science at utah valley university (graduated 2020) author has 63 answers and 130.1k answer views 4 y related what is the molecular geometry of sef4?
In the o2 molecule, both oxygen atoms equally share the 4 electrons that make up the double bond between them. In a single oxygen atom, there are eight protons, eight electrons, and eight neutrons.
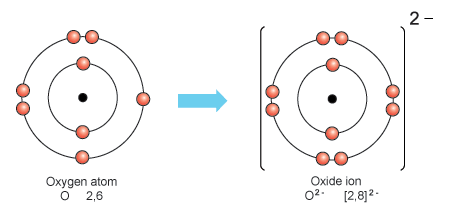
How Many Protons And How Many Electrons Are In An Oxygen Ion That Has A -2 Charge? | Socratic

How Many Protons And How Many Electrons Are In An Oxygen Ion That Has A - 2 Charge?