Therefore, for a hydrogen atom to be stable, it only needs two electrons. The double hydrogen anion h − − does not exist as a stable species.
How Many Electrons Does Hydrogen Have? - Quora
Hydrogen atoms have one electron each.
Does hydrogen have electrons. There are no electrons in a hydrogen ion. Each atom has a different number of electrons than uranium. What is the electron of hydrogen?
Hydrogen is represented by the symbol “h”. Hydrogen atoms are composed of one proton and one electron. So by gaining a electron it can.
The electron configuration for hydrogen indicates that it only has one shell. Hydrogen is the most abundant element in the. Rare hydrogen isotopes such as deuterium.
The atom would have no electrons. Why does hydrogen only have 1 proton? The one means that it has 1 proton, and the number of protons and electrons is.
A hydrogen atom can lose its only electron in an ionic bond. These atoms are the lightest possible since they only have one electron and one proton. Determine the valence shell and calculate the total electrons the third step is.
Does hydrogen want 2 valence electrons?. Does h have 2 electrons? The h+ ion has no electrons and is a bare charge with only about 1/64 000 of the radius of a hydrogen atom.
Only one electron is needed for a hydrogen atom. In general, the first shell can hold 2 electrons, the second can. (it occurs as a resonance, with a lifetime of 23 ns though.) if you tried to add another electron to a h − ion,.
Hydrogen atom can gain as well as lose an electron. In addition to its mass, hydrogen has more electrons than uranium. It may be because hydrogen was one of the first elements to exist in the universe.
In a neutral atom number of electrons=number of protons=atomic number. No, uranium has far more electrons. We know that hydrogen atoms have only an electron.
It has only one electron in its 1s orbital. See answer (1) best answer. The vast majority of hydrogen atoms have no neutrons;
That is, the first shell of hydrogen has an electron. To be electrically neutral, it must have one electron. Hydrogen can have any number of electrons and still be hydrogen.
Why does hydrogen have only one proton? Explain that the two electrons in the. If hydrogen has 1 electron it can only have 1 outer shell (or orbital) as an electron cannot by split across shells.
They combine into molecules of two so that they can share two electrons (one each). Actually the correct answer is. One s orbital can have maximum 2 electron.
Hydrogen is the first element on the periodic tale, with an atomic number of 1. Can an atom not have any electrons?
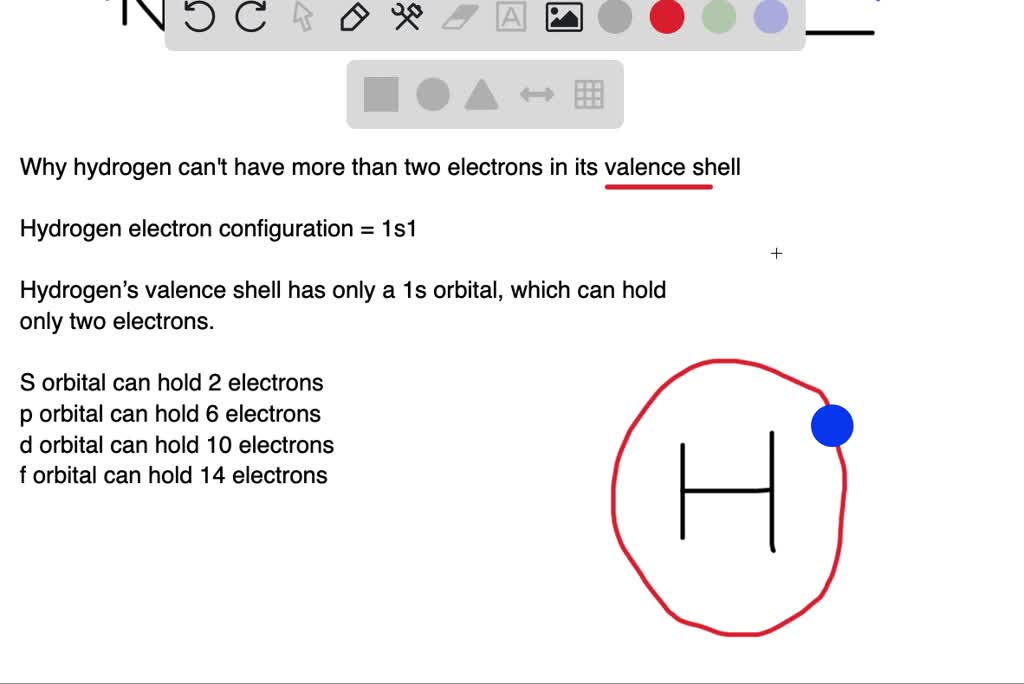
Solved:why Can't Hydrogen Have More Than Two Electrons In Its Valence Shell?