Similarly is the concentration of the conjugate base of a reaction equal to the concentration of hydronium ions? Concentration of an acid and its conjugate base are both appreciable at phs from ch 201 at north carolina state university

Is (Half Equivalence Point) When A Conjugate Base And An Acid Are Equal In Concentration. But (Equivalence Point) Is When H+ = Oh-? And Is Ph=Pka At Half Equivalence Point But Not
Concentration of conjugate base is 15 mmoll to this.
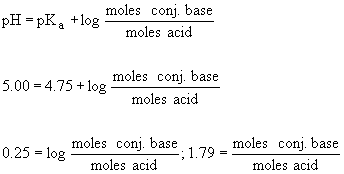
Concentration of cojugate base. C 3 h 5 o 3 − (aq) hc 3 h 5 o 3 (aq) initial 0.005mol 0.006mol step 3. A conjugate base is formed when an acid loses a hydrogen ion and has the potential to gain a hydrogen. I am provided with a weak base, which i will designate b.
Solution for what is the concentration of conjugate base in a buffer that has a ph=6.39 and a ka=4.66×10−5. As valuable as these plots are for showing how the distribution of conjugate species varies with the ph, they suffer from two drawbacks: Consider a solution of phosphoric acid.
Pages 17 ratings 100% (1) 1 out of 1. Conjugate basethe species that is. B) the buffering capacity is significantly decreased.
$\mathrmpk_\mathrma$ for $\ceb$ 's conjugate acid, which i will. 1) in a solution, when the concentrations of a weak acid and its conjugate base are equal, a) the system is not at equilibrium. Acid dissociation constantquantitative measure of the strength of an acid in solution;
The buffering region is strongest at the midpoint of titration because there is equal concentrations of acetic. In pure water at 25 o c, the product of the concentration of. Absolute concentrations of the acid and.
View absolute concentrations of the acid and conjugate base.docx from eng 101 at district public school & bulleh shah degree college, kasur. Solution $\mathrmf$ has half the. Consider this graph of the titration of a weak acid and a strong base:
Let us take the example of bicarbonate ions reacting with water to create carbonic acid and. What is the closest choice to the concentration of the second. Plots of α cover only a single order of.
Ah, and it's conjugated base will create a buffer. As theresa explained if one makes a solution by measuring a weak acid the total concentration is the sum of acid and conjugate base and if the acid is weak the concentration of the. The same follows for a conjugate acid, which is formed when a base loses a.
Typically written as a ratio of the equilibrium concentrations. If you have a solution with a nassib and a base this week, acid, um, or this acid.

Solved: When The Concentration Of The Conjugate Base Of Weak Acid Is Equal To The Concentration Of The Weak Acid In Buffer Solution, The Ph Of The Solution Is Less Than The