It appears that the same set of formal charges can be achieved. Science chemistry q&a library draw the lewis structure for the chlorite ion (cio2) with minimized formal charges.
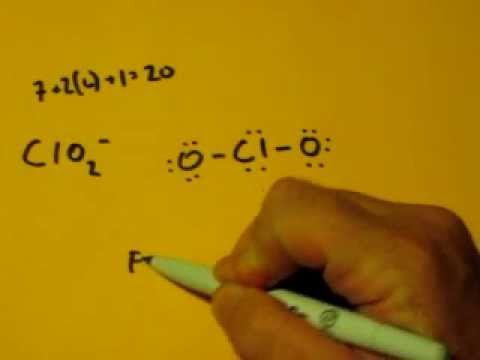
Lewis Dot Structure Of Clo2- (Chlorite Ion) - Youtube
You have 20 valence electrons in your trial structure.
Chlorite ion lewis structure. 82% (28 ratings) transcribed image text: How many charges in atoms of chlorate ion. Therefore, shape of ion is trigonal pyramidal.
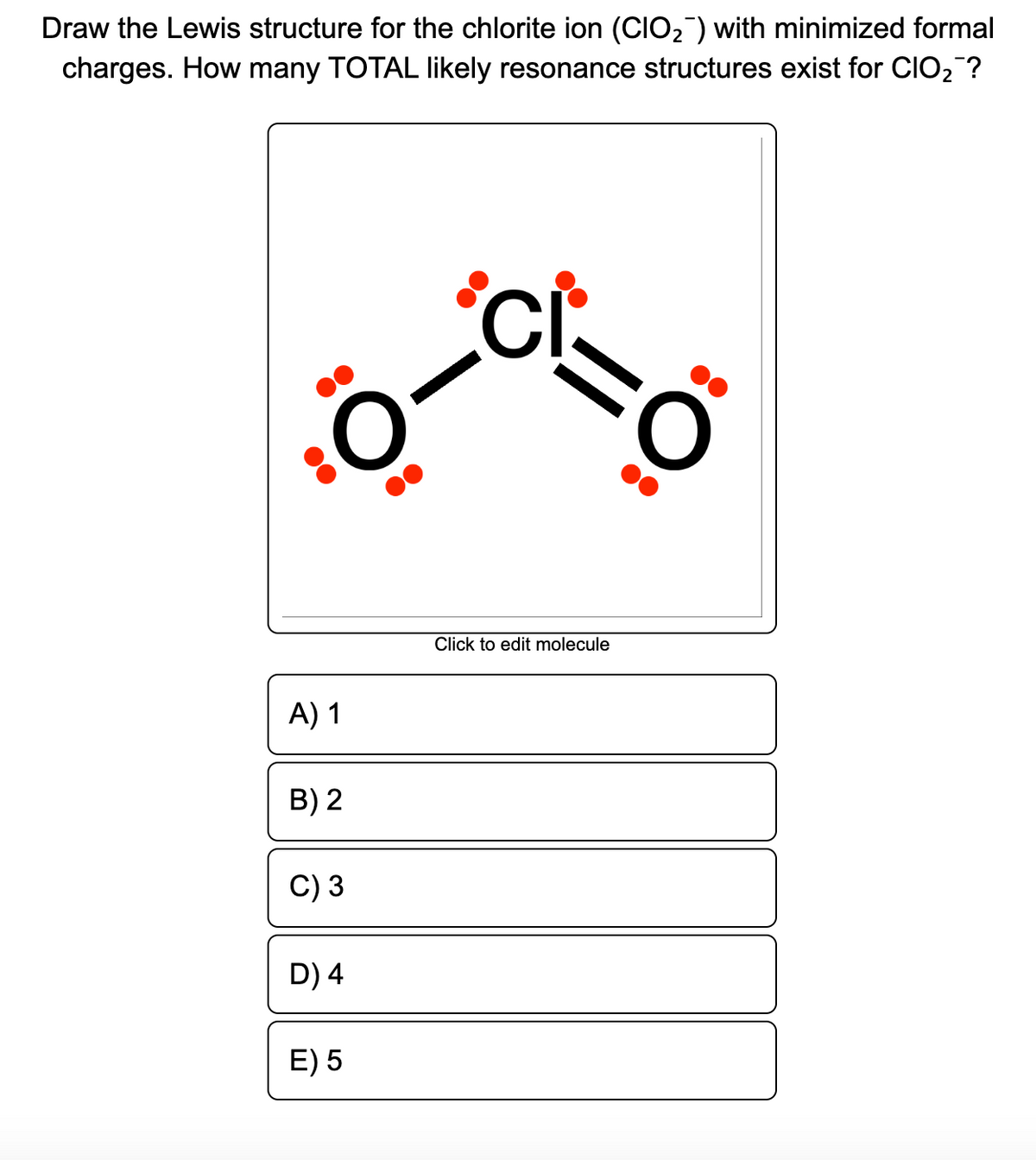
How many total likely resonance structures exist for. (assign lone pairs, radical electrons, and atomic charges where appropriate.) this problem has been solved! It is bent because of the lone pairs of valence electrons on.
70 more lewis dot structures. In order to find the total. In this ion, the chlorine atom does follow the octet rule, unlike clo 3−, or clo 4−.
From the lewis structure, it is clear that chlorine dioxide or chlorite ion is a triatomic molecule that is bent in shape. Assign a formal charge to each. So, cl is the central atom.
You can find the procedure here. Assigning one double bond to the structure makes for formal charges of o ( − 1) − c l ( 0) = o ( 0) o ( − 1) = c l ( 0) − o ( − 1). Remember that the negative sign counts as one valence electron.

Clo2- Lewis Structure, Molecular Geometry, Hybridization- | Molecular Geometry, Molecular, Covalent Bonding
