So, there are three regions of. For this you need the atomic (molecular) mass of ch4o.

Ch3Oh Lewis Structure , Molecular Geometry And Shape - Geometry Of Molecules
Generally, the lone pairs in the molecule distort the shape of the molecule, which changes the molecule’s bond angles.

Ch4o molecular geometry. In such page, we additionally have number of images out there. As a result they will be pushed down giving. Such as png, jpg, animated gifs, pic art, symbol, blackandwhite, pix, etc.
The ch4 molecule will have 109.5° bond angles as there is no distortion in its shape. Within the structure of methane, hydrogen atoms form a 109.5 degree angle with the carbon atom. If you're searching for ch4o molecular geometry subject, you have visit the ideal web.
Based on vsepr theory (valence shell electron pair repulsion theory) these electrons will repel the electron clouds of the two oxygen atoms on the end. What is the lewis structure molecular geometry polar? See the answer see the answer see the answer done loading.
As it releases more light and heat on burning, it is preferred more than coal, fossil fuel, or gasoline for energy production. This problem has been solved! These atoms repel each other in a way that the final shape of ch4 appears like tetrahedral.
The carbon (c) central atom is located in the center of the tetrahedron, while the four hydrogens (h) atoms are located on the vertices. We have got 5 picture about ch4o molecular geometry images, photos, pictures, backgrounds, and more. How do you calculate the number of moles in 0.998 grams of ch4o?
Ch4, commonly known as methane, is a tetrahedral structure with four hydrogen atoms forming around a central carbon atom. Ch3oh the carbon is tetrahedral electron geometry and tetrahedral molecular geometry the oxygen is tetrahedral electron geometry and bent molecular geometry. Take the number of grams and divide it.
The molecular geometry of h2o is bent. The molecular geometry of ch2o is trigonal planar as the carbon central atom has no lone pair and is attached to the two hydrogens atoms and one oxygen atom with the help of two single bonds and one double bond. Ch4 lewis structure, molecular geometry, and hybridization methane or ch4 is a naturally occurring gas and relatively abundant on the earth, making it an economically efficient fuel.
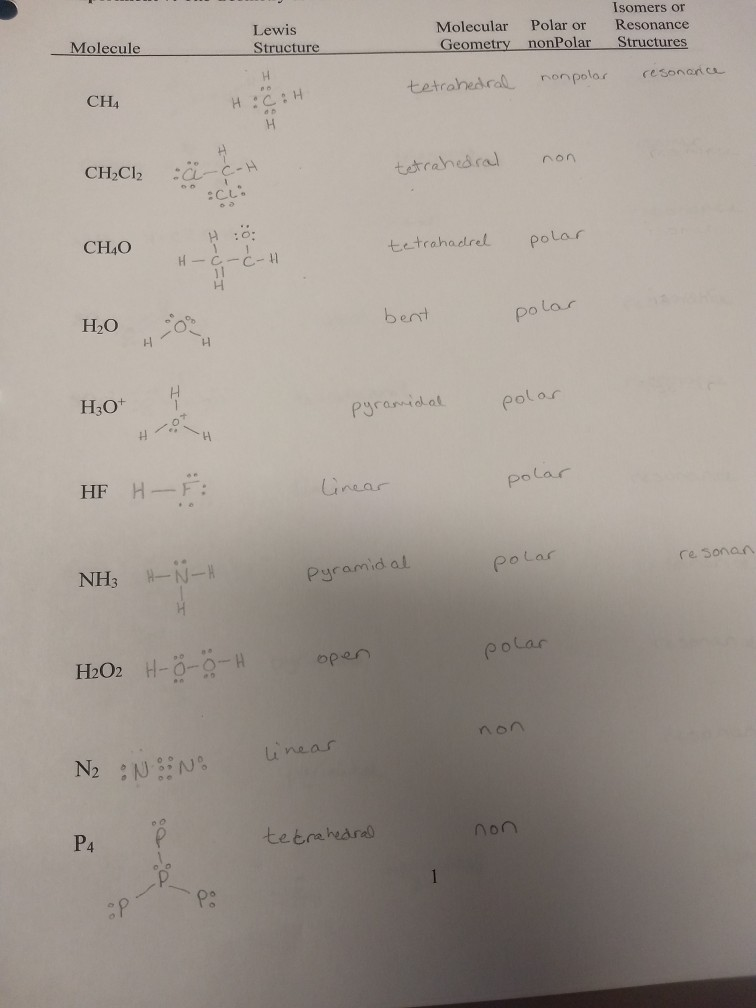
Species ch4 ch2cl2 ch4o h2o h3o+ hf nh3 h2o2 n2 p4 c2h4 lewis structure. Pictorially, this structure resembles a pyramid in shape, with all four corners equidistant from the center. The molecular geometry of ch4 is tetrahedral.
Study guides chemistry 20 cards to name a monatomic anion change the suffix of the element's name to the electron geometry of a water molecule is even though the molecular geometry.
Lewis Structure, Hybridization, And Molecular Geometry Of Ch3Oh | By Dipesh Malhotra | Medium
Solved Isomers Or Lewis Structure Molecule Molecular Polar | Chegg.com