When equilibrium is obtained, delta g = 0, so delta g0 = rtln k, ie. Every reaction will be spontaneous in.

The Relationship Between Free Energy And The Equilibrium Constant - Video & Lesson Transcript | Study.com
The first is look up the δ g values on a gibbs free energy table (delta g) and then take the δ g of the products minus the δ g of the reactants.
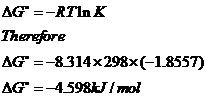
Calculating delta g degree. Δg = δg∘ +rt lnq. Δ h is in the. Delta g = delta g0 rtlnq, where q = product reactants expression.
Calculate delta g degree for the reaction. Gibbs free energy, denoted g, combines enthalpy and entropy into a single value. The relationship between δg and pressure is:
If we know the standard state free energy change, g o , for a chemical process at some temperature t, we can calculate the equilibrium constant for the process at that temperature. 335 subscribers this video is about calculating delta g of the reaction for the decomposition of carbon tetrachloride gas at 25 degrees celsius. When the divider is broken, the ammonium nitrate.
Calculate delta g degree for the reaction. Delta = change in price of asset / change in price of underlying. Instant cold packs, often used to ice athletic injuries on the field, contain ammonium nitrate and water separated by a thin plastic divider.
The second way to calculate δ g is to use a formula that involves enthalpy, temperature, and entropy. Δ g is in the units joules (j). 7 0 1 volt at 2 5 ∘ c before its use.
From my understanding, the naught refers to standard conditions, making me think that the only difference between the two. You are free to use this image on your website, templates, etc, please provide us with an attribution. Ecu^2 + /cu = 0.34v
Where q is the reaction quotient, that in case of a reaction involving gaseous reactants and. The formula for delta is: Delta h is the change in enthalpy during a chemical reaction, which may possibly be positive or negative.
Solution for calculate delta g degrees and kp for the following equilibrium at 25°c. 18.3 gibbs free energy and. Calculate the minimum mass of n a o h required to be added in r h s to consume all the h + present in r h s of the cell of emf + 0.
Answer of calculate delta g degrees and kp for the following equilibrium at 25°c. This is the same technique. The deviation of delta g from delta g0 is given by:
Enthalpy change says a lot about whether a chemical reaction is positive. I'm just a little confused about these two things. The change in free energy, δg, is equal to the sum of the enthalpy plus the product of the.

Oneclass: Calculate Delta G Naught Rxn And E Naught Cell For A Redox Reaction Where N=2 And K = 22
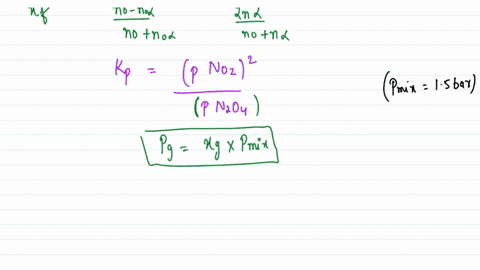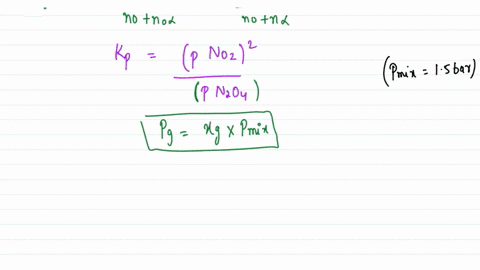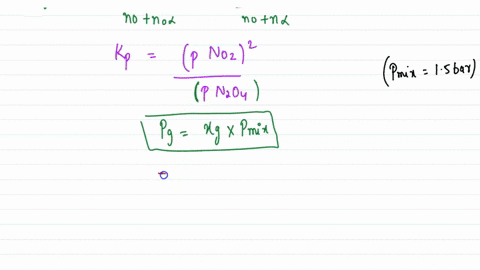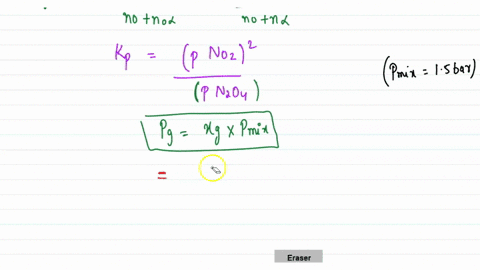
Solved:assuming That \Delta H_F^\Circ Is Constant In The Interval 275 \MathrmK -600 . K, Calculate \Delta G^\Circ For The Process \Left(\MathrmH_2 \MathrmO, G, 298 \MathrmK\Right) \Rightarrow \Left(\MathrmH_2 \MathrmO, G, 600 ...