This is due to the difference in distances between br and the four. Br has 4 bonded electron.
Brf4- Molecular Geometry - Youtube
Angles aren't perfect 120 and 90 etc as in vsepr theory lone pairs take up 'more space' than bonding pairs.

Brf4- bond angle. The electron geometry for the xenon tetrafluroide is also provided. The fluorine atoms will each have 3 lone pairs. Bromine trifluoride (brf3) is an interhalogen pale yellow liquid with a strong odor.
Tell me about the atomic charges, dipole moment, bond lengths, angles, bond orders, molecular orbital energies, or total energy. Fluorine atoms on the equatorial positions have the bond angles. The reason why a fluorine ion exists in this.
Sulfuric acid dissolves it, although it interacts strongly with water and organic molecules. This is due to the difference in distances between. What is the bond angles of brf4?
What is the f b f bond angle in b f 3 ? According to this website, the structure of bromine pentafluoride confirmed your claims. The correct answer is 90.84, 89.20 and 179.9 degrees.
Verify by checking the lewis dot configuration. The atoms themselves make no distinction between normal covalent and dative covalent bonds. According to the vsepr theory, the molecular geometry of brf5 is square pyramidal and its electron geometry is octahedral because bromine being the central atom has five bonds.
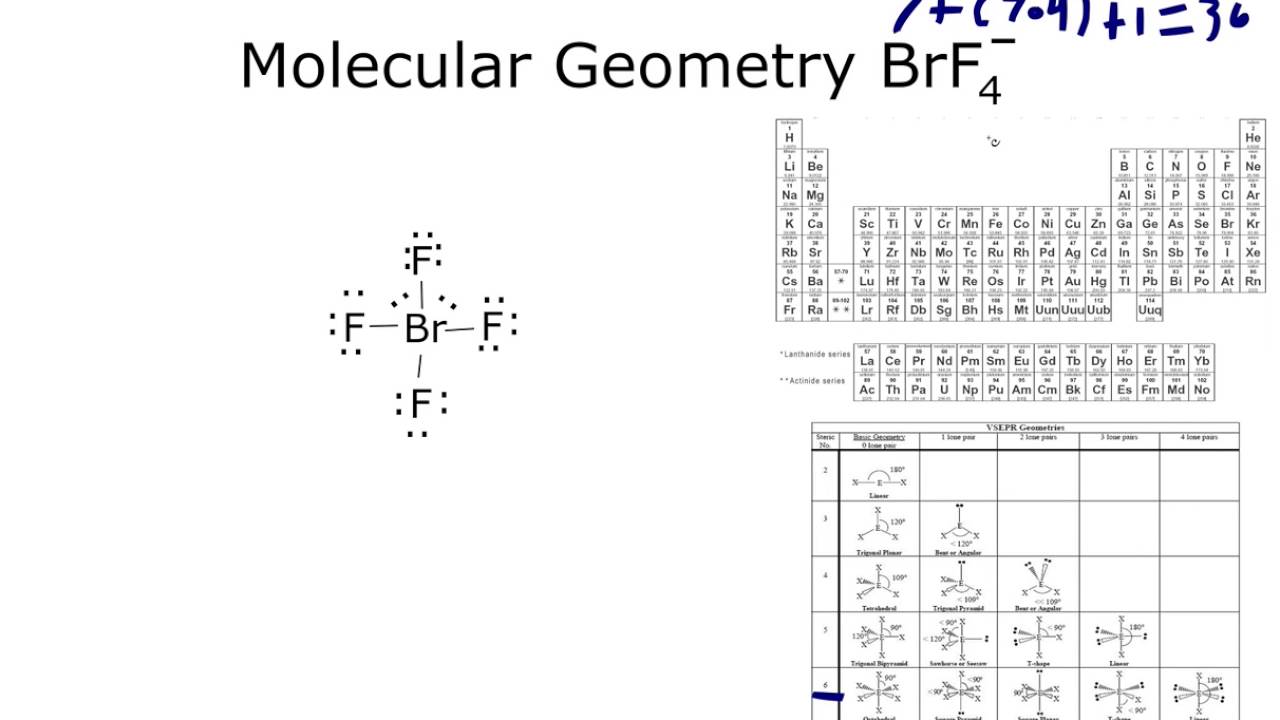
Molecular geometry is tetrahedral.and ∠f −b −f ≡ 109.5∘. The bromine atom will be bonded to each of the four fluorine atoms via single bonds for a total of 8 of the 36 valence electrons. To start, br atom has 7 valence electrons.
What is the bond angle for the molecule sicl2f2? 4 of those electrons come from br and have already been counted. For both n h 3 and bf − 4 electronic geometry is tetrahedral to a first approximation.
The other 4 come from the f. The central sulfur atom forms four bonds with the neighboring fluorine atoms and has one lone pair of electrons. This can be rationalised by considering that lone pairs are localised on the.
These are just ideas to help us explain where the. The ideal bond angle for the xenon tetrafluroide is 90 or 180° since it has a square planar molecular geometry. Sp3d2, this accounts for all 6 electron domains found around the central br atom.
The correct answer is 90.84, 89.20 and 179.9 degrees. Most think is 90 and 180 degrees. Having a straw i.e, colorless to yellow appearance,.
In brf4+, the fluorine bridges are equatorial and compress this angle. Most think is 90 and 180 degrees. (a) 60 (b) 90 (c) 109.5 (d) 120 (e) 180 ;

Chemical Bonding And Molecular Structure (Ch. 10) Molecular Structure General Summary -- Structure And Bonding Concepts Octet Rule Vsepr Theory Electronegativity. - Ppt Download