Bond orders \(1, 2, 3\) signifies the presence of single, double, triple bonds,. What is the bond order of o2?

Molecular Orbital Theory -- Homodiatomics Use The Molecular Orbital Model To Fully Describe The Bonding In O2+, O2, O2-, And O22-. Determine Which Of The Following Statements Are True And Which Are
O2 (2+) is isoelectronic with n2, which also has bond order 3.
Bond order of o2+. The value of bond order also predicts the number of bonds in the molecule. As the atomic number of o2 is 18. We review their content and use your feedback to.
You should notice that bond order is indirectly proportional to the length of. H2 , o2 , f2 have zero dipole moment. In a neutral o 2 molecule, there are a total of 12 valence shell electrons shared between the bonded atoms.
Experts are tested by chegg as specialists in their subject area. So for finding the bond order o2 will be considered as the number of potassium are 19, the nmber of electrons will be 38. The bond order of o2 the bond order is the number of bonds present between two atoms in a molecule or ion.
We have, bond order = 1 2 [ 10 − 5] = 2.5. In this case we will consider o2 also. 1 answer mika dec 16, 2014 remember that the bond order is the number of bonds between two atoms.
Bond order = 1 2(10−6) = 4 2 = 2 b o n d o r d e r = 1. Hence one σ bond and 1 ∏ bond is formed between 2 o atom. Asked dec 21, 2020 in chemical bonding by taashi ( 15.8k points)
The bond order of o2 (2+), where you’ve removed 2 electrons from an antibonding orbital is 3. For a diatomic molecule e.g o2 has bond order two because o=o is a double bond. B o n d o r d e r ( b.
Bond order n b n a =. Various molecular properties can be understood by this concept such as variation in bond length and bond enthalpies. Therefore, the bond order of o 2 + is 2.5.
Solution verified by toppr correct option is d) as bond order increases bond length decreases the bond order of species are: The molecular configuration of o 2− is as σ1s 2,σ ∗1s 2,σ2s 2,σ ∗2s 2,σ ∗2p z2,π2p y2≈π2p z2,π ∗2p y2≈π ∗2p z1 bond order = 2n b−n a = 210−7 ∴ bond order = 1.5 solve any question of. O) = 1 2 [ a − b] where a = number of electrons in bonding molecular orbitals.
Bond order is the number of bonds. How does the bond length between the oxygen atoms in. For o2+molecule, an electron is removed from * 2py orbital.
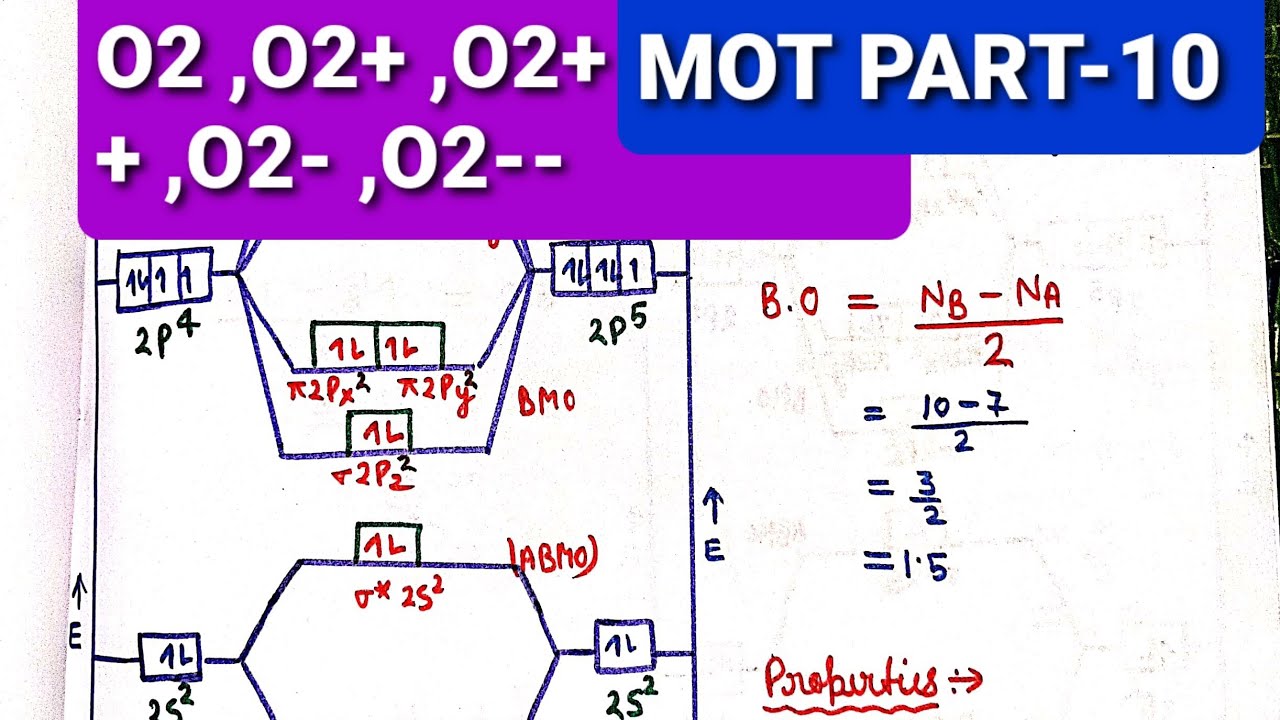
Mo Diagram O2+ , O2 2+ ,O2- ,O2 2- (Preparation Of Gate /Csir Net/Uset/Set Exam ) - Youtube