An aromatic compound is a compound that contains one or more rings with pi electrons that are delocalized all the way around the ring or rings. Acnh island design ideas 2021;
Naming Aromatic Compounds - Chemistry Libretexts
Aromatic rings (also known as aromatic compounds or arenes) are hydrocarbons which contain benzene, or some other related ring structure.
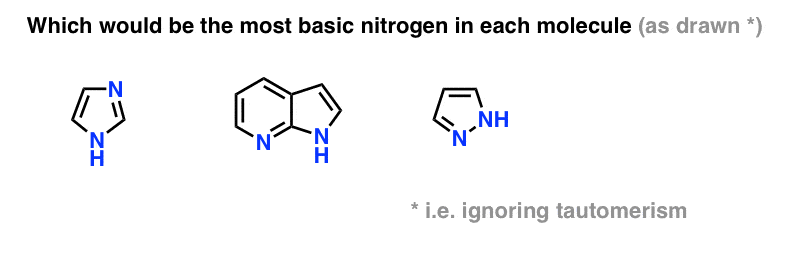
Are aromatic rings more basic then methyl. Dear student aniline is an aromatic amine and it has a benzene ring which is electron withdrawing in nature. Aromatic rings are common structural components of polymers. Consider the lone pairs of electrons.
You get a phenyl group, c 6 h 5, by removing a hydrogen from a benzene ring, c 6 h 6. Electrophilic aromatic substitution with arenediazonium salts practice 1. You get a phenyl group, c 6 h 5, by removing a hydrogen from a benzene ring, c 6 h 6.
Actually , in case of aromatic compounds , electron pair may get involve (not in all cases, only possible in. Reactions proceed much slower in rings bearing. The electron pair of this c − h bond then becomes part of.
Aliphatic amines are more basic than aromatic amines; So previously we learned that hello jin's our weekly deactivating. Benzene, c 6 h 6, is often drawn as a ring of.
Identify the following molecules as aromatic, antiaromatic or nonaromatic. Aromatic rings are common structural components of polymers. No, usually aromatic compounds are less basic and aliphatic compunds.
Because of this, the lone pair of electrons on nitrogen is not readily. Methyl group in the aromatic ring in β tocopherol the methyl groups are in the from foas aafs 3314 at tunku abdul rahman university college, kuala lumpur. The aromatic ring has two substituents a methyl group activator and a nitro from chem 2112 at temple university.
That means that their pre bad at deactivating benzene rings so much that they actually funct… In aromatic amines, the −nh 2 group is attached to a −c 6h 5 group, which is an electron withdrawing group. So, the lone pair of electrons on nitrogen are readily available.
Remember that you get a methyl group, ch 3, by removing a hydrogen from methane, ch 4. Aliphatic amines are more basic than aromatic amines. Now, the stronger the ability of the amine to donate the electron pair, the more basic it is.
For example, ethyl amine is more basic than ammonia because the electron donating effect of the. No, usually aromatic compounds are less basic and aliphatic compunds. Remember that you get a methyl group, ch 3, by removing a hydrogen from methane, ch 4.
Actually , in case of aromatic compounds , electron pair may get involve (not in all cases, only possible in.
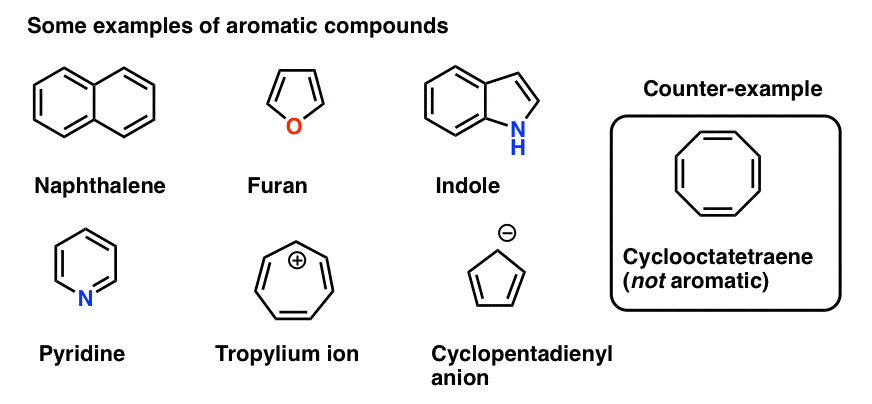
Rules For Aromaticity: The 4 Key Factors – Master Organic Chemistry
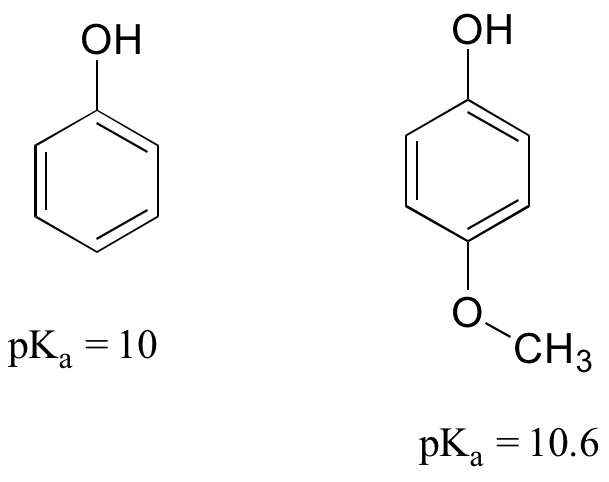
7.9: How Delocalized Electrons Affect Pka Values - Chemistry Libretexts