The chemical symbol for lithium is li. This is because the element’s atomic number is 13, reflecting the fact that it has 13 electrons and 13 protons.
Solved Question 20 An Aluminum Ion, Al 3+, Has: 13 Protons | Chegg.com
10 typically in any standard atom, we have the same number of electrons as protons.

Al+3 protons. Mercury is a chemical element with atomic number 80 which means there are 80 protons in its nucleus. See the answer determine the number of protons, neutrons and electrons for al +3: In this case, the valency of aluminum is 3.
Total number of protons in the nucleus is called the. In this video we’ll use the periodic table and a few simple rules to find the number of protons and electrons for the aluminum ion (al 3+). A proton is a positively charged particle that resides within the atomic nucleus.
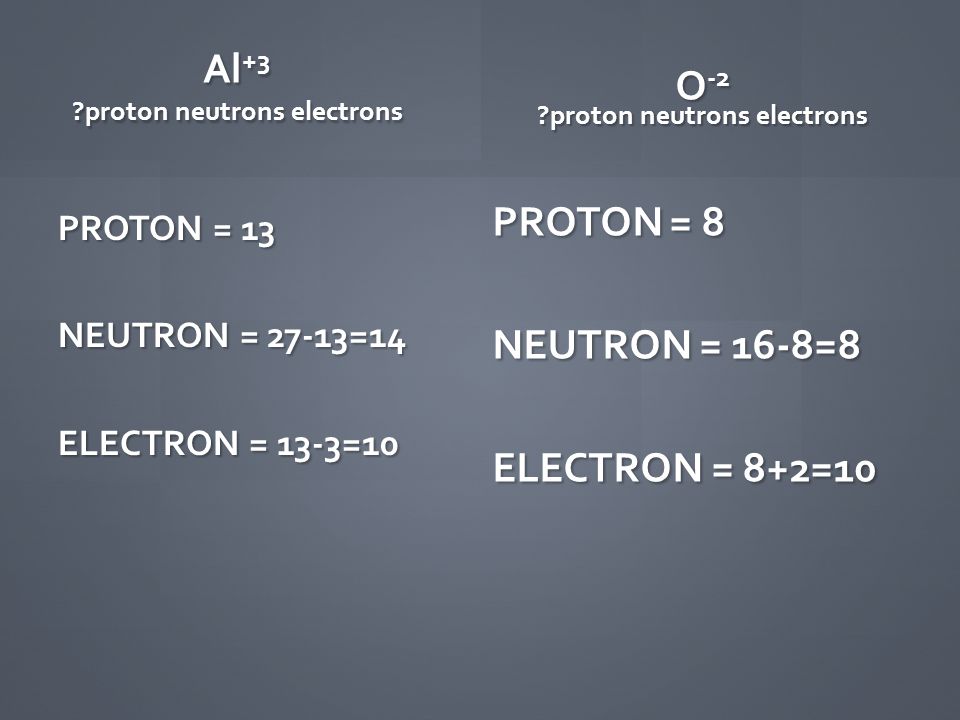
Protons = 13, neutrons = 27, electrons = 10 protons = 13, neutrons = 14, electrons = 29 protons = 13,. Clearly, there are 10 electrons associated with a single al3+ ion. The valence shell of aluminum has.
The number of protons in the atomic nucleus is what determines the atomic. Atomic number of aluminium aluminium is a chemical element with atomic number 13 which means there are 13 protons and 13 electrons in the atomic structure. Number of protons is usually equal to number of electrons, but this is not.
Now to the number of protons; We don't need any chemistry to make this determination. Now to the number of protons;
The elements that have 1, 2, or 3 electrons in the last shell donate the electrons in the last shell during bond formation. Of electrons in aluminium = 13aluminium loses 3 electrons to give al+3. Al is having a charge of +3, and this means al has lost 3 of its valence electrons.
All atoms of fluorine have 9 protons. Mike jones maed in chemistry & physics, western carolina university (graduated 1974) author has 6.2k answers and 4.6m answer views 1 y the aluminum ion. From the periodic table we can find the.
Al is having a charge of +3, and this means al has lost 3 of its valence electrons. The charge of an aluminum ion is typically 3+. 13, 10, 14 13, 14, 15 13.
Therefore we have 3 less. What would be chemical formula for a neutral compound composed of those two ions? But we have a 3+ ion here.
The difference between al and al+3 is that the ion has lost 3 electrons, therefore it has 3 more protons than electrons, hence granting it a charge of +3. The atomic number specifies the number. It will be al^ (3+).
Of protons in aluminium = 13 ∴no. Of aluminium = 13 ∴no. View (exercise 2).docx from chemistry 301 at technological university of the philippines manila.
A cation to be more specific. There is clearly no confusion between the aluminium ions because ionization enthalpy increases with increasing positive charge on the atom, as the energy required to. Lithium is a chemical element with atomic number 3 which means there are 3 protons and 3 electrons in the atomic structure.
Protons and neutrons in mercury. Chem quizzes al+3 has _ protons, _ electrons, and _ neutrons. View chem quizzes.docx from chem 110 at pennsylvania state university.
A student has 0.0555 moles of.

Jumlah Proton Elektron Neutron Dari 27 13 Al 3 + Adalah
Unit 3 – Nuclear Model Of The Atom - Ppt Video Online Download
