Neutral atom chlorine (z=17), for instance has 17 electrons,therefore, its ground state electronic configuration can be written as 1s 22s 22p 63s 23p 5. Since 1s can only hold two electrons the next 2 electrons for f go in the 2s orbital.
F- Electron Configuration (Fluoride Ion) - Youtube
Get 20% off grade+ yearly subscription →.

Flouride ion electron configuration. The fluoride ion is f− f − meaning that it has one extra electron than the neutral fluorine atom. 1s 2s 2p 3s b. The electron configuration for zn +2:
Therefore the f electron configuration will be 1s 2 2s 2 2p 5. So first, let's write the electron configuration of a fluoride atom. What is the electron configuration of fluoride ion?
The fluoride ion is a fluorine atom that has gained 1 electron. What is the difference between the two? F¯ (10) => 1s² 2s²2p⁶ next, we shall determine the electronic configuration of the element given in options to see which is the same as that of the fluoride ion (f¯).
+3 is the common oxidation state of al. Since the last shell of fluoride ion has eight electrons, the valence electrons of fluoride ion are eight. What is the electron configuration of fluoride ion?
Click here 👆 to get an answer to your question ️ the electronic configuration of fluoride ion is the same as that of a neon atom. The same as that of a potassium ion. What is the charge on fluoride ion?.
The electronic configuration of the neutral atom is thus [f] = 1s22s22p5 [ f] = 1 s 2 2 s 2 2 p 5. Fluorine f has the electron configuration of 1s22s22p5 this configuration is unstable lacking one electron to achieve a filled electron shell. It also exists in +1 and +2 oxidation states by losing one one and two electrons.
By losing the three valence electrons aluminium can attain a stable electronic configuration thereby forming al+3 ion. Medium solution verified by toppr fluoride ion is negatively charged ion with 9 protons and 10 electrons whereas neon atom is electrically neutral with 10 protons and 10 electrons. Compound formation of fluorine by valence electrons
Fluoride f −1 has the electron configuration of 1s22s22p6 this configuration is stable but leaves fluoride with a negative one charge which causes it to be reactive chemically. What is the difference betwe… The remaining five electrons will go in the 2p orbital.
Thing 1 chemistry periodic table chart The transition metals still do not end up being isoelectronic with a noble gas, but the loss. F is atomic number 9 and so has 9 electrons.
In writing the electron configuration for fluorine the first two electrons will go in the 1s orbital. The correct answer is 1 s 2 2 s 2. The same rule will apply to transition metals when forming ions.
It resembles the configuration of the nearest inert gas i.e neon. 1 s 2 2 s 2 2 p 6 \mathrm1s^2 2s^2 2p^6 1 s 2 2 s 2 2 p 6. Floride ion (f¯) simply indicates that the fluorine atom has gain an extra electron.
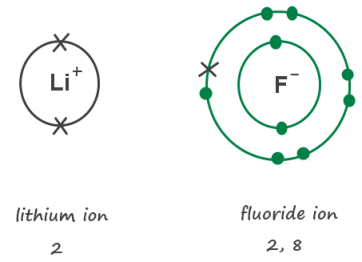
It has 2 electrons in the first shell, and 8 in the second as it gains an electron to form an ion so the configuration is 2,8. Thus, the electronic configuration of the fluoride ion (f¯) can be written as follow: Periodic table by nayab ali.
Its electronic configuration is 1s2 2s2 2p6 3s2 3p1 number of valence electrons is 3. It is an extremely reactive and the most electronegative element in chemistry. The same as that of a neon atom d.
Properties and uses of fluorine at standard conditions, it appears as a pale yellow diatomic gas. The electron configuration of a fluoride ion, f, is ____. The same as that of a neon atom.
1 s2 2 s2 2 p5. The electronic configuration of fluoride ion is the same as that of a neon atom.