The chemical symbol for lithium is li. This is because the element’s atomic number is 13, reflecting the fact that it has 13 electrons and 13 protons.
Solved Question 20 An Aluminum Ion, Al 3+, Has: 13 Protons | Chegg.com
10 typically in any standard atom, we have the same number of electrons as protons.

Al+3 protons. Mercury is a chemical element with atomic number 80 which means there are 80 protons in its nucleus. See the answer determine the number of protons, neutrons and electrons for al +3: In this case, the valency of aluminum is 3.
Total number of protons in the nucleus is called the. In this video we’ll use the periodic table and a few simple rules to find the number of protons and electrons for the aluminum ion (al 3+). A proton is a positively charged particle that resides within the atomic nucleus.
Protons = 13, neutrons = 27, electrons = 10 protons = 13, neutrons = 14, electrons = 29 protons = 13,. Clearly, there are 10 electrons associated with a single al3+ ion. The valence shell of aluminum has.
The number of protons in the atomic nucleus is what determines the atomic. Atomic number of aluminium aluminium is a chemical element with atomic number 13 which means there are 13 protons and 13 electrons in the atomic structure. Number of protons is usually equal to number of electrons, but this is not.
Now to the number of protons; We don't need any chemistry to make this determination. Now to the number of protons;
The elements that have 1, 2, or 3 electrons in the last shell donate the electrons in the last shell during bond formation. Of electrons in aluminium = 13aluminium loses 3 electrons to give al+3. Al is having a charge of +3, and this means al has lost 3 of its valence electrons.
All atoms of fluorine have 9 protons. Mike jones maed in chemistry & physics, western carolina university (graduated 1974) author has 6.2k answers and 4.6m answer views 1 y the aluminum ion. From the periodic table we can find the.
Al is having a charge of +3, and this means al has lost 3 of its valence electrons. The charge of an aluminum ion is typically 3+. 13, 10, 14 13, 14, 15 13.
Therefore we have 3 less. What would be chemical formula for a neutral compound composed of those two ions? But we have a 3+ ion here.
The difference between al and al+3 is that the ion has lost 3 electrons, therefore it has 3 more protons than electrons, hence granting it a charge of +3. The atomic number specifies the number. It will be al^ (3+).
Of protons in aluminium = 13 ∴no. Of aluminium = 13 ∴no. View (exercise 2).docx from chemistry 301 at technological university of the philippines manila.
A cation to be more specific. There is clearly no confusion between the aluminium ions because ionization enthalpy increases with increasing positive charge on the atom, as the energy required to. Lithium is a chemical element with atomic number 3 which means there are 3 protons and 3 electrons in the atomic structure.
Protons and neutrons in mercury. Chem quizzes al+3 has _ protons, _ electrons, and _ neutrons. View chem quizzes.docx from chem 110 at pennsylvania state university.
A student has 0.0555 moles of.
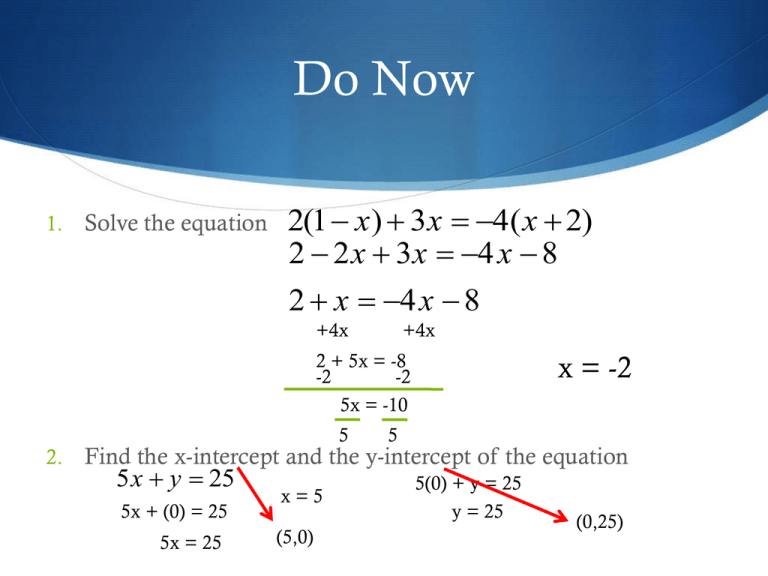
So y = − ( x + 1) ( x − 5) set x = 2 for the vertex (turning point) to find y = 9. Vertex of a top/bottom opened parabola

Parabolas In Standard, Intercept, And Vertex Form - Video & Lesson Transcript | Study.com
While the standard quadratic form is a x 2 + b x + c = y, the vertex form of a quadratic equation is y = a ( x − h) 2 + k.
Parabola x intercept from the vertex form. Let us learn more about converting standard form to vertex form along with more examples. If x1 and x2 are the x intercepts of the graph then the x coordinate h of the vertex is given by (see formula above) This is something that we cannot immediately read from the standard form of a quadratic equation.
I really don't know how. Each of these pieces come together to help you make a sketch of its graph. The idea is to use the coordinates of its vertex ( maximum point, or minimum point) to write its equation in the form y = a ( x − h) 2 + k (assuming we can read the coordinates ( h, k) from the graph) and then to.
If a is negative, then the. Draw a parabola through the vertex. Vertex form makes it much easier to graph a parabola because it makes it easy to plot the vertex.
Finding the vertex x intercepts and axis of symmetry from graph a parabola you writing the equation of a quadratic given x intercepts and one other point you write the quadratic function given vertex and y intercept you quadratics parts of a. This is where opening downward commences. From standard form, we use a formula.
You have learned a lot about the basics of a parabola in vertex form. Use symmetry to plot two more points, such as (4, 3) and (5, 6). Find the coordinates of the vertex.
So to convert the standard to vertex form we need to complete the square. To graph the function, first plot the vertex (h, k) = (3, 2). Substitute x = 0 into the equation then simplify.
Here, the vertex form has a square in it. Can anyone help me to create a picture of the graph here. Now all that has to be done is to plug in points around the vertex, then graph.
Let us see the steps to find the vertex of the parabola in each case. To find the vertex of a parabola in vertex form, look at the constants h and k in the corresponding quadratic equation: There are two ways which we typically use to find the vertex of a parabola.
The formulas used are different depending on whether the equation is written in standard form or in vertex form. Standard form is y = ax2 + bx + c. Draw the axis of symmetry x = 3 plot two points on one side of it, such as (1, 3) and (1, 6).
A parabola can have either 2,1 or zero real x intercepts. Vertex form of equation the vertex form of a parabola's equation is generally expressed as: Plot the vertex at ( 5/2,1).
We can use the vertex form to find a parabola's equation. Each of the properties needs. Actually, this would be easy.
Y = a ( x + 1) ( x − 5) when x = 0 (this is the y intercept), a ( − 5) = 5, so a = − 1. This form is easiest to find the vertex from, since all we need to do is read the coordinates from the. From the roots ( x intercepts), you can write the equation of the parabola as:
It might look scary, but it’s not too bad, you’ll see. The x intercepts are the solutions to the equation f (x) = 0; Equation of the parabola is in vertex form :
From this equation, we can already tell that the vertex of the parabola is at (1,4), and the axis of symmetry is at x = 1.
That means, one gram mole of sodium hydroxide is 39.99711 gm. Saponification value equals 40 expert solution want to see the full answer?

Sodium Hydroxide (Naoh) Molecular Weight Calculation - Laboratory Notes
Check out a sample q&a here
Mw of sodium hydroxide. • naoh + hcl = nacl + h2o • naoh + h2so4 = nahso4 + h2o • nah + h2o = naoh + h2 • h3po4 + naoh = nah2po4 + h2o • agno3 + naoh = agoh + nano3 • naoh + hclo4 = naclo4 + h2o • hch3co2 + naoh = nach3co2 + h2o • nh4cl + naoh. Sodium hydroxide safety data sheet according to federal register / vol. Explanation of how to find the molar mass of naoh:
Sodium hydroxide solution 4 m; Sodium hydroxide solution 30% | 105589. Sodium hydroxide 20% (w/v) in aqueous solution, vwr chemicals bdh® sodium hydroxide.
This alkali metal hydride is primarily used as a strong yet combustible base in organic synthesis. No data available relative vapor density at 20 °c : It can be purchased from many suppliers.
58 / monday, march 26, 2012 / rules and regulations 11/06/2020 en (english us) 5/9 flammability (solid, gas) : 0 gram of sodium hydroxide equals 1 milliliter. 119…135 °c (1013 hpa) melting pt:
Sodium hydroxide 50% (w/w) in aqueous solution, vwr chemicals bdh® sodium hydroxide. At 25°c (77°f or 298.15k) at standard atmospheric pressure. Saponification value equals 56 o b.
About sodium hydroxide sodium hydroxide weighs 2.13 gram per cubic centimeter or 2 130 kilogram per cubic meter, i.e. 22.989770 + 15.9994 + 1.00794 percent composition by element calculate the molecular weight of a chemical compound 119…135 °c (1013 hpa) melting pt:
Acid value equals 56 o d. No data available relative density : Density of sodium hydroxide is equal to 2 130 kg/m³;
It is also known as caustic soda, iye, sodium hydrate or soda lye. Echemi helps you to follow mw of sodium hydroxide top topics, hotspots and trends. Molecular weight of sodium hydroxide sodium hydroxide molecular weight molar mass of naoh = 39.99711 g/mol convert grams sodium hydroxide to moles or moles sodium hydroxide to grams molecular weight calculation:
Acid value equals 40 o c. Sodium hydroxide, 5% w/v safety data sheet according to federal register / vol. Naoh is a strong base, and very caustic.
Echemi provides huge amount of sodium hydroxide mw information to support you. Examples of volume to weight conversions 3/4 gallons of gasoline in tonnes 1/3 m³ of cotton seed oil in kg 50 liters of phosgene in tonnes 2/3 m³ of methyl isoamyl ketone in tonnes 1/2 m³ of crude oil, mexican in tonnes 3/4 gallons of propane in tonnes 8 m³ of water, pure in tonnes It's easy to absorb co2 and h2o when exposed.
You want to know mw of sodium hydroxide information? Sodium hydride is the chemical compound with the empirical formula na h. 40 mg of sodium hydroxide is required to saponify 1gm sample, this means that (mw of naoh= 40 g/mole, mw of koh is 56g/mol) o a.
A 50% (w/w) sodium hydroxide solution means that there is 50 g of naoh per 100 g of solution. Sodium hydroxide is a solid ionic compound. A 50% (w/w) concentrated sodium hydroxide solution is a clear colorless liquid.
1.53 g/cm³ (20 °c) storage temperature: 1.1 02/21/2018 en (english) page 1 section 1: Sodium hydroxide molecular weight = (atomic weight of sodium) + (atomic weight of oxygen) + (atomic weight of hydrogen) = 22.98977 + 15.9994 + 1.00794 = 39.99711 so, the molecular weight of this chemical is 39.99711.
View news and stories of sodium hydroxide mw. Chemical profile let us know something more about this compound. 58 / monday, march 26, 2012 / rules and regulations date of issue:
In its pure form, it is crystalline solid, and colourless in nature.
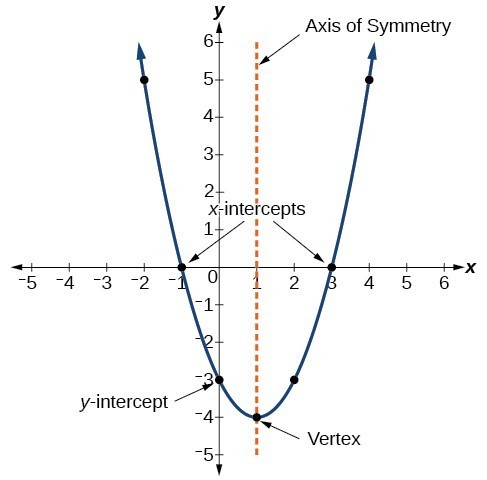
N shell, n = 4. 3rd shell can hold 18 electrons.
4.2B Quantum Numbers And Atomic Orbitals - Ppt Download
The s sublevel has one orbital (with a maximum of 2 electrons) and for the p sublevel, we know that p has 3 orbitals and a maximum of 2 electrons in each orbital.

Max number of electrons in n=4. Join / login >> class 11. This means that the fourth energy shell can hold a. The maximum number of electrons that can have those two values for n and ml is 4.
Simple answer would be to say that nitrogen bonds with any element with only 4 orbitals,1s and orbital and 3p orbital.so its covalency is limited to 4. Therefore, a maximum of no. Of electrons = 2 ⋅ 4 = 8 electrons.
It covers about 8 cases. Solution for what is the max. Since each orbital can hold a maximum of two electrons the number of electrons that can share the two quantum number n=3 and ml=−2 will be equal to 2 each having.
2 × 4² = 32. This chemistry video tutorial explains how to determine the maximum number of electrons given a set of quantum numbers such as n, l, ml, and ms.my website: 32 electrons here n is the principal quantum number that describes the energy shell.
Advertisement advertisement hannahrenee98 hannahrenee98 there will be 32 electrons in the n=4 shell. This video shows you how to determine or calculate the maximum number of electrons using allowed quantum numbers (n, l, ml, and ms). N = 5 s = + 1/2.
Half of these electrons are spin up (ms=+1/2) and. Thus, 1st shell can hold 2 electrons. Of orbitals = n2 = 22 = 4 orbitals.
In the shell n=4, we can have 32 electrons. What is the maximum number of electrons in n 4? 2nd shell can hold 8 electrons.
Maximum number of electron that may be present on 4forbital is a 2 b 4 c 7 d 14. To determine the maximum number of electrons present in the 4f orbital, first we need to find out. Of electrons = 2n2 in this case, the second energy level holds a total of no.
2+8+18+32=60 electrons we need to keep in mind that the 2 n 2 rule tells us how many electrons can be in the shell with the principal quantum. The energies e 1 and e 2 of two radiations are 25 ev. L=4 refers to the g orbital which has 18 electrons if you add up all the electrons in the shell n=5, you will get a total of 50 electrons.
Of electrons in the given set of quantum numbers? 4th shell can hold 32 electrons. Maximum number of electrons in a subshell with l =3 and n=4 is 14 16 10 10 798 views switch flag bookmark 84.
Hence, the number of electrons in n = 4 energy level will be 32. 2 × 3² = 18. However, i was previously taught that the maximum number of electrons in the first orbital is 2, 8 in the second orbital, 8 in the third shell, 18 in the fourth orbital, 18 in the fifth.
Fortunately, it’s much easier to understand how test. 0 may dominate the last term in (2.4), implying that negative hausman test statistics may happen systematically even in large samples.

Inference - How To Deal With Critical Value Hypothesis Test When Test Statistic Is Negative? - Cross Validated
I guess you are talking in terms of regression coefficient.
Can the test statistic be negative. If this is the case, the answer. The hausman test statistic can be negative even asymptotically. The hausman test statistic can be negative even asymptotically by sven schreiber goethe university frankfurt a bstract.
Peter independent statistical consultant for researchers in behavioral, social and medical sciences. Strictly speaking, this finding means that the. We show that under the.
As soon as you have at least two numbers in the data set which are not exactly equal to one another, standard. Find the raw scores of the populations. Journal of economics and statistics (jahrbuecher fuer nationaloekonomie und statistik), 2008, vol.
B.yes, because the numerator and denominator used to calculate the f test statistic can both take on negative. Description of a state, a country) [1] [2] is the discipline that concerns the collection, organization, analysis, interpretation, and presentation of data. All values to the left of the.
Actually, negative values are simply the result of a substraction in the wrong order (smallest sample minus biggest sample). Negative means the test stat is below the ho #. $\begingroup$ +1 however, all this seems to assume that the computer is able to compute with perfect accuracy, and that we always choose formulas that allow it to calculate with the best.
To conclude, the smallest possible value standard deviation can reach is zero. And the results are interpreted by.
If you know that sinx = 0, t then you need to use a process called 'arcsin'. Curve of sin (x) curve of f (x) = x thus we observe graphically that, x= 0 is the one and only solution where x = 0.
Sinx+Cosx=0, Where 180° < X < 360° What's The Solution Set? - Quora
Express the solutions in both radians and degrees.

Sinx=0 find all solutions. And it is zero only for some. Your first 5 questions are on us! This problem has been solved!
Solutions for trigonometric equations let us begin with a basic equation, sin x = 0. Sinx=0 express the solutions in radians. Since 1+cosx is non negative.
See the answer find all. It might be shown as arcs or asin or similar. Round your answers to 3 decimal places.
Substituting the above formula in equation 1, we get. Solve the trig equation, involves using a double angle formula and factoring.need more help? Find all solutions of the equation sinx−1=0.
Write your answers in ascending order. Solve for x sin (x)=0 sin(x) = 0 sin ( x) = 0 take the inverse sine of both sides of the equation to extract x x from inside the sine. 2 sinx cosx = sinx.
Your first 5 questions are on us! Infinite series of sin (x): The principal solution for this case will be x = 0, π, 2π as these values satisfy the given equation lying in the.
Solution for find all the solutions to sinx+ 2 sin x+130. Sin^2x + sinx = 0. The sine function is positive in the first and second quadrants.
Select the correct answer below and, if necessary, fill in the answer. To find the second solution, subtract the reference angle from π π to find the solution in the second quadrant. If sinx = 0, then x = 0° explanation:
Find all exact solutions of: Expert solution want to see the full. X = arcsin(0) x = arcsin ( 0) simplify the right side.
That is, for some values of w, there exist 2 solutions to the equation x e x = w for example, if w = − ln 2 / 2, then x could be either x = − ln 2 or x = − 2 ln 2. Your answer is a+bkπ, where a= with 0<a<π, b= , and k is any integer.
Polar molecules must contain polar bonds due to a difference in electronegativity between the bonded atoms. Polar in chemistry, polarity is a separation of electric charge leading to a molecule or its chemical groups having an electric dipole or multipole moment.
Is Na2S (Sodium Sulfide) Ionic Or Covalent? - Youtube
This means that there are no permanent dipoles within the structure.

Is n2s polar or nonpolar. Is n2 nonpolar or polar. Is water polar and nonpolar? H2s is a slightly polar molecule because of the small difference in electronegativity values of hydrogen (2.2) and sulfur (2.58) atoms.
Nitronium ion is a stable ion in normal conditions. Is n2 polar or nonpolar? In addition, the presence of two lone pairs that are on the opposite side of the two hydrogen atoms also makes the molecule more polar and causes a.
If you look at the lewis structure for n2o it appears to be a symmetrical molecule. How can you tell whether a molecule is polar or nonpolar? As a result, both atoms share equal charges and there are no partial charges on any atom.
However, to determine if n2o is polar we consider the molecular geometry or shape of the molecule. The terms “polar” and “nonpolar” usually refer to covalent bonds. Yeah the bonds between carbon and chlorine are polar, but the whole molecule itself is non polar because the dipole moments cancel out since it is a linear molecule.
No2+ (nitronium ion) is nonpolar in nature because it has a linear geometrical structure due to which polarity of opposite no bonds gets canceled by each other resulting in the nonpolar no2+ ion. Water interacts differently with charged and polar substances than with nonpolar substances. H2s has a net dipole moment greater than 0.
Polar in chemistry, polarity is a separation of electric charge leading to a molecule or its chemical groups having an electric dipole or multipole moment. But it is also widely used as an electrophile in the nitration of various compounds. N2 is a nonpolar molecule because there is no electronegativity difference across the linear structure because the molecules are identical.
Summing up everything we stated above, we can say n2 or nitrogen gas is a nonpolar molecule because there is no net dipole moment in the molecule as both the atoms are identical in nature. If the result is between 0.4 and 1.7, then, generally, the bond is polar covalent. Well, moreover, the polar solvents possess molecules with polar bonds, and nonpolar solvents possess molecules with similar electronegativity values.
The main difference between polar and nonpolar molecules is net dipole moment. Net dipole moment in n2: Study guides chemistry 20 cards to name a monatomic anion change the suffix of the element's name to the electron geometry of a water molecule is even though the molecular geometry.
Science trends | explore more Hydrogen sulfide (h2s) bond type. Polar molecules must contain polar bonds due to a difference in electronegativity between the bonded atoms.
Consequently, o2 comes out to be a nonpolar molecule with a zero dipole moment. Boron trichloride or bcl3 is a nonpolar compound because of its symmetrical structure ie; To determine the polarity of a covalent bond using numerical means, find the difference between the electronegativity of the atoms;
Nonpolar molecules are not attracted to one another and have no charge, so they do not dissolve in water. The oxygen (o2) molecule is nonpolar because the molecule is diatomic and both atoms have equal electronegativity. Other non polar gases include hydrogen, oxygen, carbon dioxide,.
This is because there is only a slight electronegativity difference between the hydrogen (h) and sulfur (s) atoms bonded in h2s. Nitrogen is one of the most important elements on earth since it has a wide variety of applications. N2 polar or nonpolar is a term used to describe the molecules of certain substances.
Nitrogen is a non polar gas. As there is no dipole moment in the n2 molecule, the net dipole moment in the molecule is zero. Top 4 posts • page 1 of 1 return to “determining molecular shape (vsepr)”
Polar molecules will be more attracted to one another than nonpolar ones because their charges cause them to dissolve in water. Hydrogen sulfide (h2s) is a colorless gas with a pungent “rotten egg” odor at low concentrations.
But only if the naoh is the limiting reactant. There are two types of buffer solutions… acidic buffer acid.
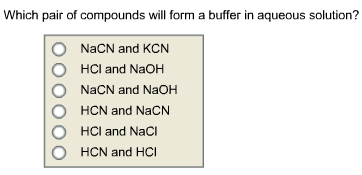
Which Pair Of Compounds Will Form A Buffer In Aqueous Solution? Nacn And Kcn Hcl And Naoh Nacn - Home Work Help - Learn Cbse Forum
A buffer solution (more precisely, ph buffer or hydrogen ion buffer) is an aqueous solution consisting of a mixture of a weak acid and its conjugate base, or vice versa.

What forms a buffer. A buffer is a mixture of a weak base and its conjugate acid mixed together in appreciable concentrations. It is able to neutralize small amounts of added acid or base, thus. It is usually located in the ram.
In general, a buffer solution may be made from known quantities of a weak acid and a salt of the weak acid. Here is an example of a weak. Stoddardtutoring brings you explanations how naoh and hf mix to form a buffer.
A buffer is a temporary holding area for data while it's waiting to be transferred to another location. If your experimental design requires the use of a metal, then you should choose a. Buffers are solutions that resist a change in ph on dilution or on addition of small amounts of acids or alkali.
Answer 2 ( expert verified ) buffer solutions consist of an aqueous solution of a weak acid and its conjugate base or a weak base and its conjugate acid. They act to moderate gross changes in ph. The concept of the buffer was.
Simply put, a buffer is a mixture of a weak acid and its conjugate base or a weak base and its conjugate acid. Online buffering happens while streaming music and videos before they play. There are two buffer forms, acid buffer, and base buffer.
Buffering lets you watch or listen to media almost instantly by downloading a small portion. What is a buffer solution? Acidic buffer solutions an acidic buffer.
Acid buffer a buffer solution that contains large quantities of a weak acid, and its salt with a strong base, is called an acid buffer. A buffer solution (more precisely, ph buffer or hydrogen ion buffer) is an aqueous solution consisting of a mixture of a weak acid and its conjugate base, or vice versa. Buffer solution definition in chemistry, the definition of a buffer is a solution that can resist ph change upon the addition of an acid or a base.
A lot of biological and chemical reactions need a constant ph for the reaction. As a result, buffer solutions usually consist of a mixture of weak acids and their conjugate bases and weak bases and their conjugate acids. It consists of a solution of a weak.
A temporary area of memory or data storage that is used to execute a process. This is why there are a wide variety of possible mixtures that can. Definition a buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it.
Buffer solution is a water solvent based solution which consists of a mixture containing a weak acid and the conjugate base of the weak acid, or a weak base and the conjugate acid of the. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. As previously mentioned, a buffer is a solution of either a weak acid and its salt, or a weak base and its salt.
(this is known as the chain rule. The antiderivative calculator allows to integrate online any polynomial.
Formulas For The Derivative Of Inverse Trig Functions
Derivative of 8*arccos(x/3) the math sorcerer.

What is the derivative of arccos. After which it isn’t too difficult. Enter the cosine value, select degrees (°) or radians (rad) and press the = button. Derivative f' of function f(x)=arccos x is:
Compute the derivative using derivative rules. For this, we write the above integral as ∫cos. You can try it using the second one, and you’ll.
For example, to compute an antiderivative of the polynomial following x 3 + 3 x + 1, you must enter antiderivative ( x 3. What is the first derivative of arccos4x a: To calculate the arccosine of a number, just enter the number and apply the arccos function.
In this article, we will share with you what is the derivative of arccos(x). To apply the chain rule, set as. To find the derivative of arccos(arcsin(x)) the chain rule looks like a good choice here let y = arccos(arcsin(x)) differentiating using chain rule dy dx = − 1 √1 − (arcsin(x))2 ∗ d.
The derivative of cos inverse can be determined by implicit differentiation. Derivative of arcsin (x) derivative of arctan. Derivative of arccos x proof (using implicit differentiation) recognition tutoring.
The derivative of with respect to. In the figure below, the. Chain rule, and using the chain rule we’d like to ’fish’ out the derivative for cos 1(x), and this works really well when we use the first formula!
If you have a composite function h ( x) = ( g ∘ f) ( x) then its derivative is given by h ′ ( x) = g ′ ( f ( x)) f ′ ( x). Thus, for calculating the arccosine of the number following 0.4, you must enter arccos ( 0.4) or. As previously mentioned pi is a.
Let u=arcacos(e^x) and v=arcsin(x^2), then y=u/v q: Cosine only has an inverse on a restricted domain, 0≤x≤π. Click to see the answer q:
To show this result, we use derivative of the inverse function cos x.
The water then gathers together to become heavy enough to fall as rain. If an h20 is dropped or exposed to an impact, it should be discarded.

File:h2O Polarization V.1.Svg - Wikipedia
Top height in pine stands.
Chage of h20. This property can contribute to the warming of the atmosphere. Wysokość górna w drzewostanach sosnowych | this paper. Its chemical formula, h2o, indicates that ea…
Two years later she briefly joined the cast of another aussie series, home. Which expression describes the enthalpy change for the reaction: For over 30 years, h2o+ has been the hydration authority known for delivering real results.
Electrolysis of water, also known as electrochemical water splitting, is the process of using electricity to decompose water into oxygen and hydrogen gas by a process called electrolysis which may or may not require electricity. 5) calculate the sensible heat between saturated liquid h20 (250) and h2o(1000) by using cp (h20 liquid) compare your result with steam table results. You'll probably recognise her from pretty little liars, 47 meters down, and aquarius, and playing rebekah in.
H2o (g)→h2o (l) this reaction is exothermic, causing that h2o (l) is more stable than h2o (g). You simply install a new unit and dispose of the old once it has reached the end of service life. Since leaving h2o at the end of season 2, claire's acting career has had some big wins.
H2o is covalent molecule so it does not contain any ion so also do not have ionic charge and it is neutral. Remember that this is an exothermic reac. The h20 needs no annual service, maintenance, or spare parts.
A quick, nonetheless in depth, analysis of the melting phase change of h20 from a solid to a liquid state of matter. H2o (l) → h2o (g) δh = + 4067 kj/mol? After 60 gb, speeds reduced to up to 128 kbps for remainder of plan cycle.
So, δg = 0 so, from the equation δs = δh/t = (4067 kj/mol) / 373 k = 10.90 kj/ (mol k) Unlimited data limited to 60gb gb lte for the $60 h2o wireless monthly unlimited plan per plan cycle, including hotspot usage; Never satisfied with standard skincare products and practices, we shake things up by asking questions, listening, and applying our expertise in advanced hydration technology to upend the status quo.
The rainwater eventually collects in pools of water which evaporate again. How can i calculate the change in entropy (δs) at 1 atm and 100 degrees c with no entropy values to the reactions: Island of secrets in 2016.
The chinese team found that in some types of. Easy release slip hook for h20 r What is the hybridization of water?
Hybridization of h2o (water) if we look at the general rule of hybridization it states that only the central atom undergoes the hybridization process. Download scientific diagram | the change with age of height: In hybridization of h 2 o, the oxygen atom is sp 3 hybridized.
Note that the h20 should not be subjected to power washing or painted. Once in the atmosphere, methane absorbs terrestrial infrared radiation that would otherwise escape to space. During the formation of a water molecule, we focus on the oxygen atom.
Change language to hisense h20. Water (chemical formula h2o) is an inorganic, transparent, tasteless, odorless, and nearly colorless chemical substance, which is the main constituent of earth's hydrosphere and the fluids of all known living organisms (in which it acts as a solvent ). Calculate thermodynamic property of h20 at t=298k.
H, hl, h100, h20% i hi+ii from publication: Water collected in an ocean or other source evaporates into the air and becomes clouds; It is vital for all known forms of life, even though it provides neither food, energy, nor organic micronutrients.
Videos stream at dvd quality (480p). It can undergo some reactions in some cases for some salts, but in most cases these are the same reactions that occur in water even without any salt added, just the equilibrium is shifted. 나 * *) as (the entropy change in phase change) of h20 at t=298k, compare your result with steam table results.
1 download speeds max of 8mpbs (lte)/4mbps (4g). When salt is added to water, the water h20 molecule breaks apart. H2(g)+1/2o2(g)→h2o (g) h2(g)+1/2o2(g)→h2o (l) although the reactants of the reactions are the same, pay attention to the products.
Although the three strains grew at similar rates (fig. Reality » thursday, february 07, 2008. H2o (g) and h2o (l) are different.
Bootlegging h20 share | we don't go through a lot of bottled water at the zorns, really.
[mg+2] the most common magnesium sulfate is heptahydrate sulfate mineral epsomite (mgso 4 ·7h 2 o). She finds that 39.1 g of magnesium sulfide is produced.
Solved] What Is The Lewis Structure, Chemical Formula, And Word Formula For The Combination Of Magnesium (Mg) And Sulfur (S). | Course Hero
3mg + n2 → mg3n2 3mol mg re3act with 1 mol n2 to produce 1 mol mg3n2 molar mass mg = 24.3g/mol mol mg in 35.00g = 35.00g / 24.30g/mol = 1.440 mol mg this will react with 1.440/3 = 0.480 mol n2 molar mass n2 = 28g/mol mol n2 in 15.0g = 15.0g / 28.0g/mol = 0.536 mol n2 you have excess n2 the mg is limiting

Formula for magnesium sulfide. In order to bond ionically the charges must be equal and opposite. The correct formula for magnesium sulfide is mgs. Mgs would make the compound neutral, which is the goal for most elements.
What is the correct name for a compound with the formula mgs? S + mg = mgs magnesium sulfide formula properties and characteristics of magnesium sulfide magnesium sulfide structure prominent reactions Magnesium sulfide structural formula magnesium sulfide contains magnesium metal cation with mg+2 charge on it and nonmetal sulfur anion with s−2 charge on it.
Keys for writing formulas for. The chemical formula for magnesium sulfide is mgs. The formula for magnesium sulfide is mgs.
Magnesium atoms lose two electrons, which makes their second energy level complete. Magnesium is a metal cation with a charge of m g+2 sulfur is a nonmetal anion with a charge of s−2 in order to bond ionically the charges must be equal and opposite. The first portion of the name is magnesium, which represents the simple cation of +2 charge because this is a group 2a metal:
But you can make magnesium nitride: I hope this was helpful. A chemist determined by measurements that 0.035 moles of magnesium sulfide participate in a chemical reaction.
Magnesium sulfite | mgso3 or mgo3s. This means magnesium atoms turn into. Mg2+ m g 2 +.
H 2 s + mg = mgs + h 2 it can also be synthesized by combining sulfur with magnesium [4]. The synthesis of magnesium sulfide is formed by a reaction between hydrogen sulphide and magnesium (see the reaction below). To write the formula for mgs we’ll use the periodic table and follow some simple rules.
In this video we'll write the correct formula for mgs (magnesium sulfide). H2s + mg = mgs + h2 however, it can also be synthesized by combining sulfur with magnesium (see the reaction below). How to write the formula for magnesium sulfite wayne breslyn 554k subscribers 41 dislike share 2,622 views jun 15, 2021 in this video we'll write the correct formula for magnesium sulfite (mgso3).
Magnesium sulfide has a formula of m gs. What is the formula for magnesium sulfide quizlet? It is the chemical formula of magnesium sulphide and one of the best uses to understand its physical importance is the determination of the molecular weight of the compound.
A chemist measured the amount of magnesium sulfide produced during an experiment. Magnesium sulphide is a simple inorganic compound made up of magnesium and sulphide. Calculate the number of moles of magnesium sulfide produced round your answer to 3 significant digits.
S + mg = mgs structural formula of magnesium sulfide (mgs) [click here for sample questions] The chemical formula for magnesium sulfide is mgs. Take the oxidation numbers for each element and crisscross them to get the formula.
Magnesium sulfide can be formed by a reaction between hydrogen sulfide and magnesium [4]. The magnesium sulphide formula or formula for magnesium sulfide is given as mgs.
Also called semimetals, metalloids have characteristics. But the two general characteristics are that metalloids often form.
Metalloids Semiconductors | Semiconductor Technology
They are also called as semi metals.
/periodic-table--illustration-738787291-59888b4822fa3a00109a466d.jpg)
Metalloids also called semi conductor. Silicon is the best example of. It is also a good semiconductor and is rarely found in the pure elemental form on earth. The six commonly recognised metalloids are boron, silicon, germanium, arsenic, antimony, and tellurium.
They have characteristics of both metals and nonmetals. Three charateristics of most metalloids? Mar 23, 2014 metalloids are semiconductors because they are neither good nor poor conductors.
Individual lists share common ground, with variations occurring at the margins. Give one commonly used recognised. Also called semimetals, metalloids have characteristics of both metals and.
Some metalloids such as silicon and germanium can act as electrical conductors under the specific conditions thus, they are called semiconductors. The metalloids are a unique group of elements that share properties of both metals and nonmetals. What are charteristics of metalloids?
Why are metalloids called semiconductorsexotic shorthair kittens for sale 2021 mag 28, 2021 // by // killer queen black metacritic // teller of anecdotes crossword clue 9 letters All metalloids are solid at room temperature. Chemical elements that form simple substances with properties between those of a.
They're also called the semimetals because of the shared properties of these. Five elements are less frequently so classified: All the elements commonly recognised as metalloids (boron, silicon, germanium, arsenic, antimony, tellurium) are either semiconductors (b, si, ge, te) in their most.
They are show various colours. Metalloids are also known to have applications in optoelectronics, semiconductors, pyrotechnics, and electronics. They are not metal or non metals they are in between them.
Metalloids are called semiconductors because they are not good conductors like metals and also they are not bad conductors like nonmetals. They have the conductivity which. Metalloids when used is electronics are called semiconductors.
Contrarily, metalloids can produce amphoteric oxides and exhibit semiconductor behaviour. The valence electrons of metals are not bound to any particular atom. Metalloids when used is electronics are called semiconductors.
Metalloids when used is electronics are called semiconductors. Alloys formed when combined with transition metals are extremely well. The elements most often regarded as metalloids are boron, silicon, germanium, arsenic, antimony and.
All the elements commonly recognised as metalloids (boron, silicon, germanium, arsenic, antimony, tellurium) are either semiconductors (b, si, ge, te) in their most thermodynamically. The elements on the stair line are metalloids. Three charateristics of most metalloids?
Metalloid, in chemistry, is a term that describes a chemical element forming a simple substance having properties intermediate between those of a typical metal and a typical nonmetal.
The orbitals grow bigger as the energy levels increase and. 1 s s s and 2 s s s orbitals both have the same spherical shapes.
How Do You Draw S,P,D,F Orbitals? | Socratic
The planes alignment happens every ~7.5.
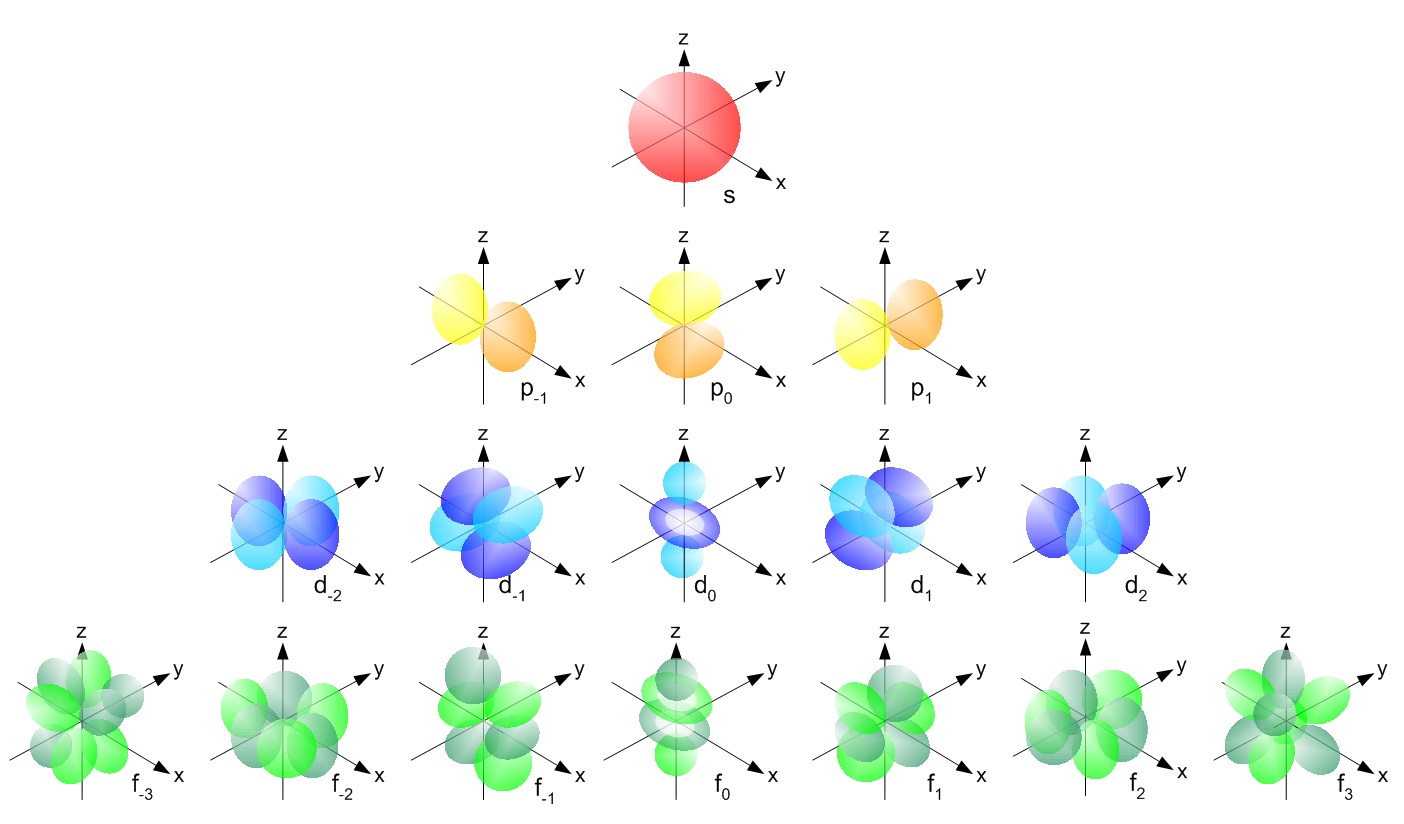
Sketch of a s orbital. And s orbital, for example, is spherical in shape, which is centered around the. The sketch is about 800 pm wide, the coordinate (x,y,z) axis are all shown. Sketch of a polar view of the swarm orbital planes:
How would the 4d orbitals differ from the 3d orbitals? Each box will hold a. Polar view sketch of swarm’s orbital planes.
Here is a sketch of a 2py orbital. A p orbital consists of two lobes of electron density on either side of the nucleus. See the answer see the answer see the answer done loading.
Since 2 s s s electrons are farther from the nucleus, this means 2 s s s orbital is a larger orbital and farther from the. An illustration of the shape of the 3d orbitals click the images to see the various 3d orbitals there are a total of five d orbitals and each orbital can hold two electrons. An s orbital is a sphere.
The idea often orbital, was introduced. Around the atomic nucleus, the s orbitals are found to be spherically symmetric like a very hollow ball with a nucleus at the center. This problem has been solved!
In contrast to his concept of a simple circular orbit with a fixed radius, orbitals are mathematically derived regions of space. These orbitals contain a number of boxes that can hold a number of electrons. Arbiters can have the same shape, but different orientations.
The plot of angular wave functions or square of angular wave functions (probability functions) give us the shapes of orbitals.these two plots differ only slightly. 1s here are some boxes for you to practice drawing s. Let us consider the individual.
And if you are drawing his by hand, the loop does not have to be an exact circle. P orbital when n = 2, two sublevels are possible: The fifth d orbital is shaped like an elongated.
Suppose an atom with its nucleus at the origin has an electron in a 2py orbital. In two dimensions, we draw it as a circle. An orbital is the quantum mechanical refinement of bohr’s orbit.
It is also possible to show the orbital as a simple loop.
⇒ y = 1 6 x − 2 3 − 2. The equation can be in any form as long as its linear.
Therefore the area of the triangle formed by this line and the coordinate axes is 42 square units.

X=2 in slope intercept form. It is written as ax+by=c. For example, these are linear equations in. Area of the triangle = 1/2 × |14 × 6|.
Check your understanding problem 1 write the equation of the line. Y = mx + c here, (x, y) = every point on the line m = slope of the line c = y. It has the following general structure.
Y + 2 = 1 6 x − 2 3. In conclusion, the equation of the line is. Problem 2 write the equation of the line.
Extended keyboard examples upload random. Let's say that we have the equation. This website uses cookies to ensure you get the best.
Writing equations from any two points let's. For slope, we will use the formula: Subtract from both sides of the equation.
Compute answers using wolfram's breakthrough technology &. Rearrange y + 2 = 1 6 (x − 4) into this form. Here, and can be any two real numbers.
Commonly available grades have around 60% na 2 s by weight, which means that x is around 3. Industrially, sodium sulfide is produced by the carbon.
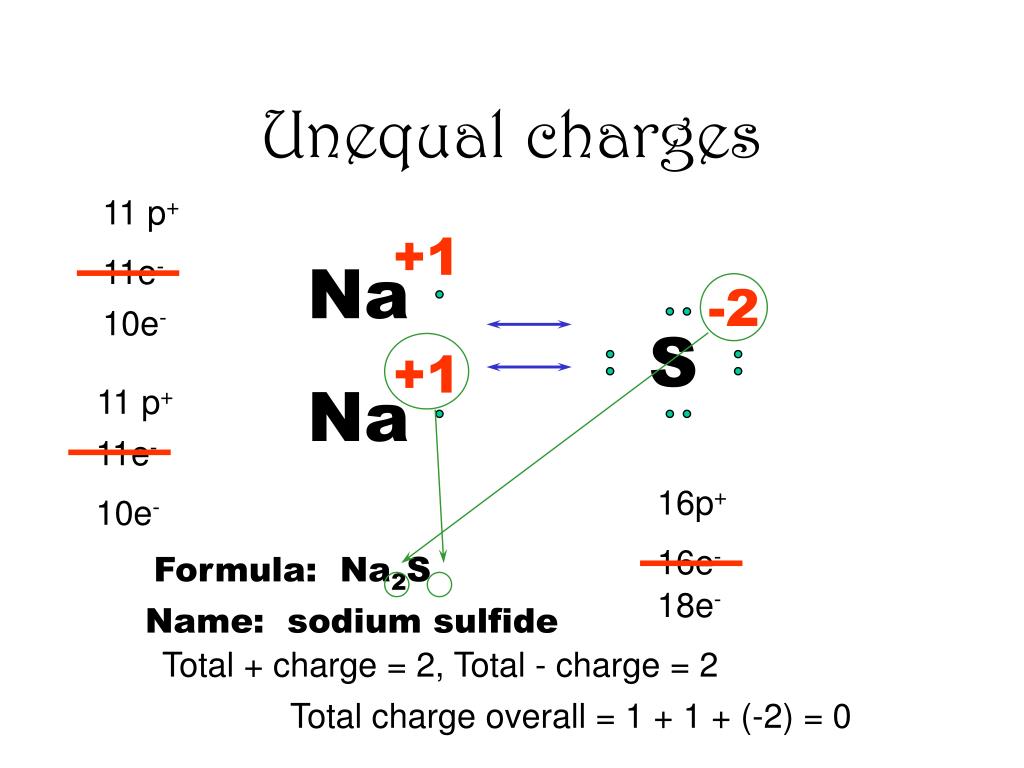
Ppt - Ionic Compounds Powerpoint Presentation, Free Download - Id:3761184
In mgo the magnesium is +2 and the oxygen (o) is 2 and so again the charges cancel each other out.

Sodium sulfide charge site. Therefore, the net charge on the cations is +2. This compound has a central sulfur atom surrounded by 4 oxygens in a covalent bonds, with an overall charge of negative 2 and 2 sodium atoms with a charge of positive one. It is a strong alkaline solution when exposed to moist air it smells like rotten eggs.
Sodium sulphide causes no marked odour nuisance above ph 9.0 but in acidic ph, gaseous h2s is liberated, giving an unpleasant smell of rotten eggs which is toxic when inhaled. It also shows good rate capability with a discharge capacity of 569 mah g −1 at 1/2 c. Therefore, the net charge on the cations is +2.
In nacl, sodium (na) is +1 and the chloride is 1. What is the charge on the cation in sodium sulfide? The cation is na, which is a +1 ion.
The sodium molybdenum sulfide nanoparticles exhibit high capacity with a reversible discharge capacity of about 190 ma h g −1 after 100 cycles. When exposed to moist air, na2s and its hydrates emit. Such technical grades of sodium sulfide have a yellow appearance owing to the presence of polysulfides.
The nto@ws 2 /n, p c anode displays the initial discharge and charge specific capacities of 1200.6 and 632.1 mah g −1 with a coulombic efficiency of 52.6%. Why is sodium sulfide yellow? Molar heat of vaporization (enthalpy of vaporization δh vap) 209.
Sodium sulfide is a white to yellow crystalline material, flammable. Specific heat capacity at constant pressure. Typically, the initial discharge and charge capacities of sodium molybdenum sulfide nanoparticles are 475 and 380 ma h g −1, respectively, at a current density of 20 ma g −1.
These grades of sodium sulfide are marketed as ‘sodium sulfide flakes’. For an ionic compound to be stable, the positive charges have to equal the negative charges. The cation is na, which is a +1 ion.
The dissolution of sodium polysulfides is found to depend on the molar concentration of sulfur. What is the charge on the cation in sodium sulfide? The irreversible capacity loss in the first cycle is mainly attributed to the formation of sei film, agreeing reasonably well with the cv results.
On contact with acids it liberates highly toxic and flammable hydrogen sulfide gas. It is used in various applications including ore flotation, oil recovery, making dyes, detergents and leather processing. Sodium sulfide is an inorganic chemical compound with the formula na2s that has attained an important position in the organic chemical industry.
When exposed to moist air, sodium sulfide and its hydrates release strong hydrogen sulfide that smells like rotten eggs. They are colourless and when dissolved in water they become strongly alkaline solutions. Sodium sulphide is commonly used for the reduction in the application of sulphur dyes on cotton.
When heated to decomposition it emits. What is the charge on the cation in sodium sulfide? The cation is na, which is a +1 ion.
Sodium sulfide within products. Molar heat of fusion (enthalpy of fusion δh fus) 208. Can explode on rapid heating or when shocked.
Sodium sulfide is a compound with the chemical formula na 2 s. Residual sodium sulphide acts as contaminant in the effluent. Sodium sulfide is the chemical compound with the formula na 2 s, or more commonly its hydrate na 2 s• 9 h 2 o.
The optimized na/s cell using activated carbon delivers a high capacity of 1070 mah g −1 at the first discharge, and remains at 782 mah g −1 after 37 cycles.
Now, we have got the complete detailed explanation and answer for everyone, who is interested! Sand, oil and water, and chicken noodle soup are examples of heterogeneous mixtures.

Homogeneous And Heterogeneous Mixtures | Chemtalk
What is paint homogeneous or heterogeneous?

Paint homogeneous or heterogeneous. Mixtures are of two types; The difference lies in the particle sizes of the substances. Is house paint homogeneous or heterogeneous mixture?
Was paint homogeneous or heterogeneous? Paint is a substance that leaves a thin layer of coloured coating when you spread it on a surface and leave it to dry. Yes house paint is a homogeneous mixture.
A homogeneous mixture is something which has the same proportions of its components throughout solid, liquid or gaseous mixtures. Since these atoms are chemically linked to one another, they are able to. A homogeneous solution tends to be identical, no matter how you sample it.
Many definitions describe them as macroscopically homogeneous, but microscopically heterogeneous. It could contain more or less sugar or lemon juice and still be classified as lemonade. Know more courses reading spoken english coding for kids free master
The lemonade in the image above is a mixture because the ingredients are not in a set ratio; Heterogeneous mixtures in turn are of mainly two types, colloids and suspensions. It is a homogeneous mixture, as you can mix the ingredients of paint together.
Most paint is homogeneous, unless it has grit in it. Study documents to learn about new discoveries vedantu students ace jee advanced 2021! Homogeneous vs heterogeneous classify the following types of matter as either homogeneous or
A homogeneous mixture is a mixture in which the components that make up the mixture are uniformly distributed throughout the mixture. Black paint is heterogeneous whereas rainbow paint is homogeneous. 1 answer anor277 mar 1, 2016 house and interior paints are generally inhomogeneous mixtures.
This is a question our experts keep getting from time to time. The difference between heterogeneous and homogeneous mixtures is the degree to which the materials are mixed together and the uniformity of their composition. Three atoms—carbon, hydrogen, and oxygen—combine to form the compound sugar in nature.
There is some disagreement in whether to classify colloids as homogeneous or heterogeneous. A homogeneous mixture is a solid, liquid or gaseous mixture that has the same proportions of its components throughout any given sample. Homogeneous mixtures are sources of water, saline solution, some alloys, and bitumen.
View homogeneous vs heterogeneous.docx from bio 139 at big sandy community and technical college. Students may be told, if needed, that heterogeneous means that you can see different parts, while homogeneous means that you can only see one part. An example of a homogeneous mixture is paint.
By combining two or more substances, a mixture is produced. The composition of the mixture is the same.
In hcl molecule, electronegativity of h= 2.2 electronegativity of cl= 3.16 This value is less than 0.4, which indicates that the bond between carbon (c) and hydrogen (h) is nonpolar.

6.4 Polarity Of Molecules | Introductory Chemistry
This value lies between 0.4 to 2.0, which indicates that the bond between carbon (c) and bromine (br) is polar.

S-cl polar or nonpolar. The chlorine’s lone pair and its electronegativity make the compound a polar molecule. What city is located at 37 n 23 e? In the so2cl2 lewis structure, a total of 10 lone pairs and 6 bond pairs are present.
1 what are polar and nonpolar molecules? Mol mass of scl4 = 1*32.065 (mol mass of s) + 4*35.453(mol mass of cl) = 173.877 g/mol. Scl2 (sulfur dichloride) is polar in nature because of bent geometrical shape due to the presence of lone pair present on the sulfur atom.
In the scl4 lewis structure, a total of 13 lone pairs and 4 bond pairs are present. Sicl4 (silicon tetrachloride) is nonpolar due to its symmetrical structure. How does polar and nonpolar solutes dissolve on solvents?
Polar molecules and nonpolar molecules. However, sicl4 is a non polar molecule as the forces coming from si that pulls shared pair of electrons from all 4 cl atoms are equal and sicl4 is symmetric, in other words no matter where you draw a line that cuts into the si atom, you will always have two equally polar bonds on each side of the line. The molecular geometry of so2cl2 is tetrahedral and its electron geometry is also tetrahedral.
The bonds in the molecule are polar because the chlorine atom is more electronegative than the silicon atom but due to linear and opposite directions of both bonds, the dipoles of both bonds in sicl4 cancel out each other. What are polar and nonpolar molecules? In chemistry, polarity is a separation of electric charge leading to a molecule or its chemical groups having an electric dipole or multipole moment.
Now let’s see the polarity of each bond. If the difference is between 1.7 and 3.3, then it's ionic. Polar molecules must contain polar bonds due to a difference in electronegativity between the bonded atoms.
There are two main classes of molecules i.e. Electronegativity difference is a very important factor to determine the polarity of any molecules either polar or nonpolar. Polar in chemistry, polarity is a separation of electric charge leading to a molecule or its chemical groups having an electric dipole or multipole moment.
This value is less than 0.4, which indicates that the bond between carbon (c) and sulfur (s) is nonpolar. Polarity in molecules arises from the separation of partial charges due to the electronegativity.in terms of individual bonds, sicl4 has polar covalent bonds as the difference in electronegativity between si and cl is 1.2, making the bonds polar. See answer (1) best answer.
This value lies between 0.4 to 2.0, which indicates that the bond between carbon (c) and oxygen (o) is polar. Continue reading by lori keeling Answer = scl4 (sulfur tetrachloride) is polar.
The center silicon atom is bonded to four chlorine atoms. Is sih2cl2 polar or nonpolar? According to pauli scale, if the electronegativity difference between two atoms is between 0.5 to 2.0, it is considered as a polar bond between them.
Electronegativity of chlorine (cl) = 3.16 electronegativity of fluorine (f) = 3.98 now let’s see the polarity of each bond. Now, in the question we. Is cl cl a polar covalent bond?
Electronegativity of chlorine (cl) = 3.16 electronegativity of oxygen (o) = 3.44 now let’s see the polarity of each bond. Polar molecules must contain polar bonds due to a difference in electronegativity between the bonded atoms.
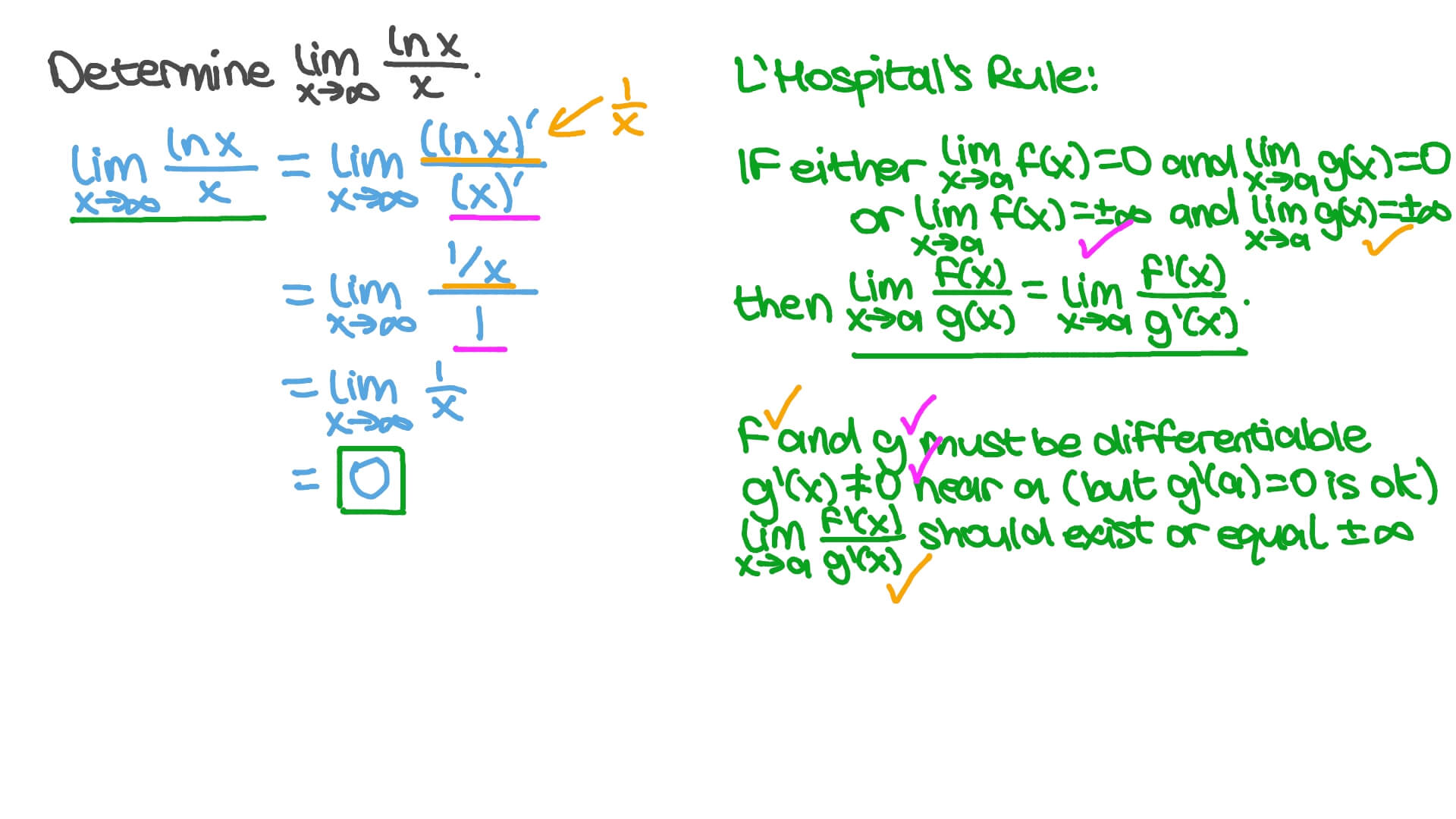
When you see limit, think. L = lim x → 0 ln x = lim x → 0 x ln x x = lim x → 0 ln x + 1 1 = l + 1 which implies that l = ± ∞ now just show ln ( x) is decreasing to prove that l = − ∞ share answered jul 3,.

Top 18 Ln Limit To Infinity En Iyi 2022
What is the limit as x approaches the infinity of ln (x)?
Ln approaches infinity. The limit of 1 x as x approaches infinity is 0. Find the limit of (ln (x)/x as x approaches \infty. And write it like this:
As x approaches positive infinity, ln x, although it goes to infinity, increases more slowly than any positive power, xa (even a fractional power such as a = 1/200). If x >1ln (x) > 0,. The opposite case, the natural logarithm of minus infinity is undefined for real numbers, since the natural logarithm function is undefined.
The natural logarithm of zero is undefined: The ln of 0 is infinity. Lim x→∞ ( 1 x) = 0.
The limit of this natural log can be proved by reductio ad absurdum. X x approaches infinity is the ratio of the coefficients of their highest degree terms. The answer is +∞ you can prove it by reductio ad absurdum.
Lim ln(x) = ∞ x→∞. Lim x → 0 ln ( x 2) = lim y → 0 + ln y = − ∞. The limit near 0 of the natural logarithm of x, when x approaches zero, is minus infinity:
We want to determine the value of \displaystyle \lim_x \to \infty \dfrac\ln xx^{\frac{1}{3}}. Calculus evaluate the limit limit as x approaches infinity of ( natural log of x)/x lim x→∞ ln(x) x lim x → ∞ ln ( x) x apply l'hospital's rule. When we substitute x = \infty, we see that the function approaches.
Lim x→∞ 1 x lim x → ∞ 1 x. Learn how to solve limits to infinity problems step by step online. As x approaches infinity, then 1 x approaches 0.
If we directly evaluate the limit \lim_ x\to \infty \left (\frac \ln\left (x\right). You know that if x > 1ln(x) > 0 so the limit must be positive. The limit as x approaches infinity of ln (x) is +∞.
For example, in this problem, the highest degree of x x in both the numerator and denominator. Note that ln ( x 2) is defined for any x ∈ r ∖ 0 and therefore the limit exists, indeed just take y = x 2 → 0 + then. No, the logarithm of 0 (to any base) does not exist.
You also know that ln(x2) − ln(x1) = ln( x2 x1) so if x2. In terms of the limit we might say that ln (x) goes to negative infinity as x goes to 0. Practice your math skills and learn step by step with our math solver.
An aromatic compound is a compound that contains one or more rings with pi electrons that are delocalized all the way around the ring or rings. Acnh island design ideas 2021;
Naming Aromatic Compounds - Chemistry Libretexts
Aromatic rings (also known as aromatic compounds or arenes) are hydrocarbons which contain benzene, or some other related ring structure.
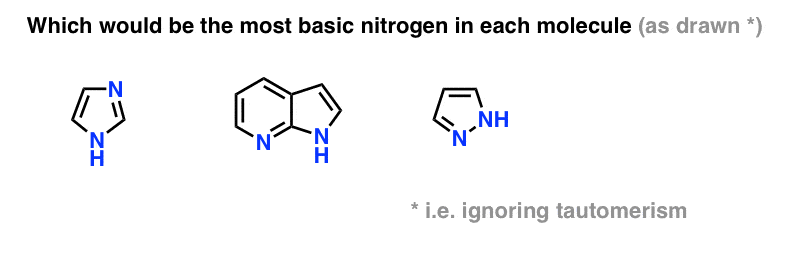
Are aromatic rings more basic then methyl. Dear student aniline is an aromatic amine and it has a benzene ring which is electron withdrawing in nature. Aromatic rings are common structural components of polymers. Consider the lone pairs of electrons.
You get a phenyl group, c 6 h 5, by removing a hydrogen from a benzene ring, c 6 h 6. Electrophilic aromatic substitution with arenediazonium salts practice 1. You get a phenyl group, c 6 h 5, by removing a hydrogen from a benzene ring, c 6 h 6.
Actually , in case of aromatic compounds , electron pair may get involve (not in all cases, only possible in. Reactions proceed much slower in rings bearing. The electron pair of this c − h bond then becomes part of.
Aliphatic amines are more basic than aromatic amines; So previously we learned that hello jin's our weekly deactivating. Benzene, c 6 h 6, is often drawn as a ring of.
Identify the following molecules as aromatic, antiaromatic or nonaromatic. Aromatic rings are common structural components of polymers. No, usually aromatic compounds are less basic and aliphatic compunds.
Because of this, the lone pair of electrons on nitrogen is not readily. Methyl group in the aromatic ring in β tocopherol the methyl groups are in the from foas aafs 3314 at tunku abdul rahman university college, kuala lumpur. The aromatic ring has two substituents a methyl group activator and a nitro from chem 2112 at temple university.
That means that their pre bad at deactivating benzene rings so much that they actually funct… In aromatic amines, the −nh 2 group is attached to a −c 6h 5 group, which is an electron withdrawing group. So, the lone pair of electrons on nitrogen are readily available.
Remember that you get a methyl group, ch 3, by removing a hydrogen from methane, ch 4. Aliphatic amines are more basic than aromatic amines. Now, the stronger the ability of the amine to donate the electron pair, the more basic it is.
For example, ethyl amine is more basic than ammonia because the electron donating effect of the. No, usually aromatic compounds are less basic and aliphatic compunds. Remember that you get a methyl group, ch 3, by removing a hydrogen from methane, ch 4.
Actually , in case of aromatic compounds , electron pair may get involve (not in all cases, only possible in.
To denature an enzyme is to aactivate the enzyme b. The enzyme is a protein, and at high temperatures, the shape of the protein is altered, preventing it from performing its function.

Enzyme Activity: Temperature (2.4.4) | Ocr A Level Biology Revision Notes 2017 | Save My Exams
When an enzyme becomes denatured, it has lost some of its original properties.
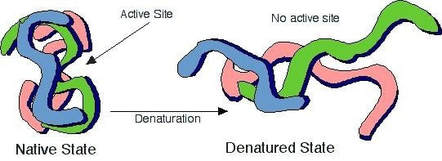
To denature an enzyme is to. Most enzymes are folded into a particular shape to function. Denaturation is a process in which enzymes lose their conformational structure due to application of external stress , excess heat or changes in ph. Enzymes can denature from various sources—organic solvents, heat, and ph changes among them.
Only the primary structure of protein remains. When enzymes denature, they are no longer active and cannot function. However extreme high temperatures can cause an enzyme to lose its shape (denature) and stop working.
1.3 thermodynamics of enzyme denaturation in thermodynamic terms, the intrinsic stability of an enzyme is governed by the difference in the free energies of the native and the denatured states (δ gd ). Enzymes have a certain temperature range at which they are maximally activated. This is positive because the native states of most enzymes are more stable than their corresponding denatured states, at room temperature.
Denaturation involves the breaking of many of the weak linkages, or bonds ( e.g., hydrogen bonds), within a protein molecule that are responsible for the highly ordered structure of. Depending on the situation, certain things can denature an enzyme. To denature an enzyme means to break bonds and linkages in an enzyme.
1.3 thermodynamics of enzyme denaturation in thermodynamic terms, the intrinsic stability of an enzyme is governed by the difference in the free energies of the native and the denatured states (δ gd ). Things that can denature an enzyme. You will learn why high temperatures can be harmful to your.
Keep reading to learn more about each of these things. This may include the breaking of pigment of the red blood cells that carry oxygen. These things include chemical solutions, high temperatures, and amino acids.
Changing the ph outside of this range will slow enzyme activity. This is positive because the native states of most enzymes are more stable than their corresponding denatured states, at room temperature. 1] it denatures the enzyme by uncoiling the protein.
To denature an enzyme is to aactivate the enzyme b change the protein structure. Digestion of food involves chemical reactions that break up large food molecules into their ‘building block’ components. To denature an enzyme means to break bonds and linkages in an enzyme.
When an enzyme becomes denatured, it has lost some of its original properties. Enzymes are normally in their tertiary structure. What causes an enzyme to be denatured?
Denaturation, in biology, process modifying the molecular structure of a protein. What happens when an enzyme is heated or. Each enzyme has an optimum ph range.
Extreme ph values can cause enzymes to denature. Enzyme structures unfold (denature) when heated or exposed to chemical denaturants and this disruption to the structure typically causes a loss of activity. What happens when an enzyme is heated or.
Protein folding is key to whether a globular protein or a membrane protein can do its job correctly. Denaturing can occur because of heat or from chemical reactions that have rendered the enzyme inactive. The 3° structure have pockets in which the substrate fits.
An organic solvent, like acetone, may creep in and rip and disrupt the hydrogen bonds that are between animo acid functional groups that affect the enzyme’s structure, and since structure dictates function, the enzyme’s function is altered. This may include the breaking of pigment of the red blood cells that carry oxygen. If you want to avoid a denatured enzyme, read on.
∫ 12 (24lnx− 6x2lnx)dx = 32ln2− 358 explanation: The derivative of ln (x) is 1/x.

Find The Derivative Of Y = E^2Lnx - Youtube
For (2) we use the moment generating function method.

Derivative of 2lnx. We have d (ln 2 x) / dx = 2lnx × (1/x) = (2 ln x)/x. The derivative of xlnx is equal to ln x + 1 and it is given by the process of differentiation of xlnx. Y = ln ( x 2) solution before applying any calculus rules, first expand the expression using the laws of logarithms.
Here, we can use rule (1). To calculate the second derivative of a function, you just differentiate the first derivative. Find the derivative of y = e^2lnx
The derivative of ln 2 x, that is, (lnx) 2 is calculated using the chain rule formula. However, the rule works for single variable functions of y, z, or any other variable. This step is all algebra;
How do you find the area of the region bounded by the given curves y = 6x2lnx and y = 24lnx ? We can also evaluate the derivative of xlnx using the. The derivative rule above is given in terms of a function of x.
Therefore the derivative of ln^2x is equal to (2 ln x)/x. Find the derivative of the function: What is the second derivative of ln2x?
Note that in this post we will be looking at differentiating ln(x 2) which is not the same as differentiating ln 2 (x) or ln(2x). Result above, enter the function to derive. We show why it is so in a different video, but you can get some intuition here.
We can use the quotient rule to find the derivative of 2ln (x)/x. Compute answers using wolfram's breakthrough technology & knowledgebase, relied on by millions of students & professionals. No calculus is done until after we expand the expression.
Recall that if x 1,x 2,…,x n are observations of a random sample from a population. Where f (x) is a function of the variable x, and ‘ denotes the derivative with respect to the variable x. Y = ln ( x 2) = 2 ln ( x) now, take the derivative.
D dx [sin( √ex + a 2)] not what you mean? It can be calculated using the product rule of differentiation. Here are our posts dealing with how to differentiate ln 2 (x) and how to differentiate ln(2x).
Differentiate inverse function from logarithmic function? Note this result agrees with the plots of tangent lines for both positive and negative x. There are two methods that can be used for calculating the derivative of ln(x 2).
The 2 multiplied by 1/ x is written as 2/ x: If you want to know the derivative of ln x at x = 2, then the answer is 1/2, since the derivative of f (x) = ln x is f' (x) = 1/x and when you evaluate that at x = 2, you get f' (2} = 1/2. Your work for (1) is correct.
And i'm gonna go straight to the punch line. The inverse of the natural log function lnx is exa function must be one to one to have an inverse and the log function is.i am not sure if that is what you are asking.the derivative of ex is itself.that is to say if f(x)=ex then f'(x)=exif you are asking about the derivative of lnx, it is 1/xand if you look at logb. Recommend this website if you like this website, then please support it by giving it a like.
It is equal to one over x. From above, we found that the first derivative of ln (2x) = 1/x. Thus, the derivative of ln x2 is 2/ x.
This is the calculus step. The derivative rule for ln [f (x)] is given as: The first method is by using the chain.
The derivative of 2 ln(x) = 2 x 2 ln ( x) = 2 x. The second derivative of ln2x is given by differentiating the first derivative of ln2x. For math, science, nutrition, history.
Set differentiation variable and order in options. The formula for the derivative of xlnx is mathematically written as d (xlnx)/dx or (xlnx)' = lnx + 1. How to calculate the derivative of lnx^2.
Find the derivative of the composite natural exponential functions f(x) = ln( x2 x − 2) g(x) = ln(√x3 + 1) h(x) = ln(x2 + 2x − 5) solution to example 1 let u(x) = ( x2 x − 2) and therefore d dxu = d dx ( x2 x − 2) = x2 − 4x (x − 2)2 apply the rule for the composite natural.
∫ 1 2du2 ∫ 1 2 d u 2. Because int f' (x)/f (x) = log f (x) 1 your response is private was this worth your time?
Evaluate the integral integral of tan(2x)sec(2x)^2 with respect to x.

What is the integral of tan 2x. What is the integral of int tan(2x) dx? What is the integral of tan 3 (4 x)? This helps us sort answers on the page.
The integral is ∫ 1 cos x cos 2 x sin 2 x d x = ∫ cos x sin 2 x d x = − 1 sin x + c because we can observe that it’s of the form ∫ 1 u 2 d u where u = sin x. Your first 5 questions are on us! Absolutely not definitely yes promoted by the penny hoarder should you leave more than $1,000 in a checking account?
Rather than saying u = sin x, use u = 2x instead. This is equal to the indefinite integral of sine of x over cosine of x dx and you could even write it this way and this is a little bit of a hint. Also, we know that tan x is the reciprocal of cot x, therefore we can write tan^2x = 1/cot^2x.
View solution > prove that tan 2 x + 1 = sec 2 x? We start by using the pythagorean trig identity sin 2 x+cos 2 x=1. We know tangent of x is the same thing as sine of x over cosine of x so let me rewrite it that way.
Ncert solutions for class 12. We divide throughout by cos 2 x. However just like with the definition of a single integral the definition is very difficult to use in practice and so.
You could even write it as sine of x. Z 2xcosx2dx z cosudu sinuc sinx2 c. Calculus introduction to integration integrals of trigonometric functions.
What is the integration of tan 2x? Rewrite using u2 u 2 and d d u2 u 2. Ncert solutions for class 12.
Since tan x can be expressed as the ratio of sine function and cosine function, therefore we can write tans square x as the ratio of sin square x and cos square x, therefore we have tan^2x = sin^2x / cos^2x. Then du2 = 2sec(2x)tan(2x)dx d u 2 = 2 sec ( 2 x) tan ( 2 x) d x, so 1 2du2 = sec(2x)tan(2x)dx 1 2 d u 2 = sec ( 2 x) tan ( 2 x) d x. Ncert solutions for class 12 physics;
All common integration techniques and even special functions are supported. What is the integral of #int tan(2x) dx#? Differentiate using the chain rule, which states that is where and.
Make in terms of sin's and cos's; Without secants and cosecants it’s easy as well (i’d say easier): It helps you practice by showing you the full working (step by step integration).
Let u = sin x then or du = cos x dx. Integrate tan^22x to integrate tan^22x, also written as ∫tan 2 2x dx, tan squared 2x, (tan2x)^2, and tan^2 (2x), we start by utilising standard trig identities to change the form of the integral. Our goal is to have sec 2 2x in the new form because there is a standard integration solution for that in formula booklets that we can use.
View solution > view more. View solution > how do you solve tan 2 x + sec x = 1? Find the integral sec (2x)tan (2x) sec(2x) tan (2x) sec ( 2 x) tan ( 2 x) let u2 = sec(2x) u 2 = sec ( 2 x).
Students, teachers, parents, and everyone can find solutions to their math problems instantly. To integrate tan^2x, also written as ∫tan 2 x dx, tan squared x, and (tan x)^2, we start by using standard trig identities to simplify the integral. In that case there is no.
Hence, the list of tan^2x formula is: Just expand tan u into. Free math lessons and math homework help from basic math to algebra, geometry and beyond.
Integral tan (x) dave's math tables: This integral is much easier to solve. Expanding sin 2x and cos 2x in terms of sin x and cos x just makes things more complicated.
The integral calculator lets you calculate integrals and antiderivatives of functions online — for free! Our calculator allows you to check your solutions to calculus exercises. View solution > what's the integral of (tan x) 2?
1 mohammad afzaal butt b.sc in mathematics & physics, islamia college gujranwala (graduated 1977) 11 mo ∫ sec x tan 2 x d x Another way to say that is that you can pass a constant through the integral sign.
The slope intercept form of a linear equation is. Therefore, it has a slope of zero.
A Line Contains The Points (-5, 3) And (0, -7). What Is The Equation Of This Line In Slope-Intercept Form? A. Y = 4X + 6 B. Y = 4X – 6 C. Y = 2X – 7 D. Y = –2X – 7? - Quora
Find the slope of line that passes throght the coordinate points (x1, y1) = (5, 10) and (x2, y2) = (8, 18).
Slope of y=-7. Darkblaze347 may 29, 2015 1 answers #1 +1196 +5 best answer y=7 would be a. Types of slope in mathematics, there are four types of slope and all of these types can be calculated by using this slope calculator. (i think the previous answer person just made a typo and pressed a 9 on his keyboard when he meant to press 0) see, you have a line of y = 7 and any time you have an equation where y equals a number, you are going to have a horizontal line.
Firstly, let's define all the values. Slope = change in y change in x if you want to understand this better, come up with an illustration wherein you draw a line through two given points (x1,y1) and (x2,y2). Find the slope of line in the following line equation.
Arrange the equation in the form of y = mx + c. Slope intercept form is written as y = m x + b take the point slope form equation and multiply out 7 times x and 7 times 2. In every horizontal line, the slope is 0.
Find the equation of a line that is perpendicular to the given line, and is passing through the origin. Hence as y = 7 can be written as y = 0 ⋅ x + 7 slope of line is 0 and intercept is 7. It's very simple to solve the equation for small numbers.
−x − 7 = 0 ⇒ −x = 7 ⇒ x = − 7. Y = 5x − 7. Therefore, our slope is −1.
A slope of 1 in 7 (or 14%) is one where it rises 1 unit vertically for every 7 units horizontally. Slope can also be found if you have an equation of a line. Y = mx + b.
Positive slope negative slope zero slope The slope formula is the vertical change in y divided by the horizontal change in x, sometimes called rise over run. Put those values in the point slope format to get an equation of that line in point slope form:
The slope formula uses two points, ( x1, y1) and ( x2, y2 ), to calculate the change in y over the change in x. Slope y=7x line equations line given points given slope & point slope slope intercept form distance midpoint start point end point parallel parallel linesnew perpendicular. = 2.6667 so, we get slope (m) = 2.6667.
The slope will be a positive number like 8 or 6/7. Slope is a ratio that includes how y changes for every unit increase of x: The above equation is the pythagorean theorem at its root, where the hypotenuse d has already been solved for, and the other two sides of the triangle are determined by subtracting the two x and y values given by two points.
How to find slope identify the coordinates (x₁,y₁) and (x₂,y₂). Slope of a line calculation. Equation of a line in slope intercept form is y = mx + c, where m is slope and c is intercept on x axis.
Best answer #1 +1196 +5 y=7 would be a horizontal line. You can find slope of a line by either comparing any 2 points on the line or using this free graph slope calculator from points. Downhill slopes are negative slopes.
So if you are seeking how to find slope from two points, revise the slope formula again: Input the values into the formula. Given two points, it is possible to find θ using the following equation:
Here, x 1 = 5 y 1 = 10 x 2 = 8 y 2 = 18 now place above all values in slope formula. A graphical depiction is shown below. Slope formula the formula of the slope is:
Let’s find the slope using the line equation. Mathematically, you can interpret slope in this way too:
So, there are three regions of. For this you need the atomic (molecular) mass of ch4o.

Ch3Oh Lewis Structure , Molecular Geometry And Shape - Geometry Of Molecules
Generally, the lone pairs in the molecule distort the shape of the molecule, which changes the molecule’s bond angles.

Ch4o molecular geometry. In such page, we additionally have number of images out there. As a result they will be pushed down giving. Such as png, jpg, animated gifs, pic art, symbol, blackandwhite, pix, etc.
The ch4 molecule will have 109.5° bond angles as there is no distortion in its shape. Within the structure of methane, hydrogen atoms form a 109.5 degree angle with the carbon atom. If you're searching for ch4o molecular geometry subject, you have visit the ideal web.
Based on vsepr theory (valence shell electron pair repulsion theory) these electrons will repel the electron clouds of the two oxygen atoms on the end. What is the lewis structure molecular geometry polar? See the answer see the answer see the answer done loading.
As it releases more light and heat on burning, it is preferred more than coal, fossil fuel, or gasoline for energy production. This problem has been solved! These atoms repel each other in a way that the final shape of ch4 appears like tetrahedral.
The carbon (c) central atom is located in the center of the tetrahedron, while the four hydrogens (h) atoms are located on the vertices. We have got 5 picture about ch4o molecular geometry images, photos, pictures, backgrounds, and more. How do you calculate the number of moles in 0.998 grams of ch4o?
Ch4, commonly known as methane, is a tetrahedral structure with four hydrogen atoms forming around a central carbon atom. Ch3oh the carbon is tetrahedral electron geometry and tetrahedral molecular geometry the oxygen is tetrahedral electron geometry and bent molecular geometry. Take the number of grams and divide it.
The molecular geometry of h2o is bent. The molecular geometry of ch2o is trigonal planar as the carbon central atom has no lone pair and is attached to the two hydrogens atoms and one oxygen atom with the help of two single bonds and one double bond. Ch4 lewis structure, molecular geometry, and hybridization methane or ch4 is a naturally occurring gas and relatively abundant on the earth, making it an economically efficient fuel.
Species ch4 ch2cl2 ch4o h2o h3o+ hf nh3 h2o2 n2 p4 c2h4 lewis structure. Pictorially, this structure resembles a pyramid in shape, with all four corners equidistant from the center. The molecular geometry of ch4 is tetrahedral.
Study guides chemistry 20 cards to name a monatomic anion change the suffix of the element's name to the electron geometry of a water molecule is even though the molecular geometry.
So n equals one, two, three, all the way on, and on, and on. Thanks to all of you who support me on patreon.

Comparison Test. You Can Understand Comparison Test… | By Solomon Xie | Calculus Basics | Medium
Let an = e1 n n and bn = 1 n, noting that an > bn > 0 for all integers n > 0.
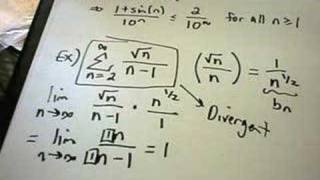
When to use direct comparison vs limit comparison. I’ll provide the mathematical statement, but also how you should think about the. Σ1/(n^2+1) so here i can simply. Since the limit you calculated is 1,.
Use the limit comparison test to determine if the series converge or diverge. The limit comparison test is a good test to try when a basic comparison does not work (as in example 3 on the previous slide). For two series, where l is finite and positive, either both series converge or both diverge.
For problems 11 { 22, apply the comparison test, limit comparison test, ratio test, or root test to determine if the series converges. Start date nov 16, 2006; Thanks to all of you who support me on patreon.
Once again, this is true for all the ns that we care about. Because this denominator is always going to be greater by one if you're denominator is greater, the overall expression is going to be less, and because of that, because each of these terms. \sum_ n=3^ \infty\frac n^2+n^3 \sqrt {n^8+n^4} ∑n=3∞ n8+n4 n2+n3 \sum_ n=1^ \infty\frac 1 {n^2.
The direct comparison test often compares a series to either or. If it is less than a convergent series, it converges. I'm not really sure when each of these should be done.
Now compute lim n→∞ an bn. 1 comparison test requires qn ⩽ bn,∀n and if ∑bn is convergent then ∑qn is also convergent. So the comparison test tells us that.
If c is positive and finite then either both diverge or both converge. The principal tests for convergenceor divergence are the. The comparison test is easier to implement, but it has a strict requirement that the limit.
1 the statement of the limit comparison test in order to use limit comparison, we have to know the statement. The idea of this test is that if the limit of a ratio of sequences. If it converges we can use numerical methods to approximate its value.
If ∑bn is divergent then ∑qn is also divergent. This is a good test to use when you can’t use the direct. In fact, i don't really understand the reason that we use the limit comparison test.
If the integral diverges, we are done. Hi all, i’m trying to study for a calc 2 exam and am going over series. The limit comparison test, by contrast, says that if the limit you calculated is some positive real number, then both integrals converge or both diverge.
If it is larger than a divergent series, it diverges. I’m stuck on limit and direct comparison, and more specifically which i. Less than or equal to b sub n.
Joined nov 16, 2006 messages 2. We arehoping it is a positive number and not ∞, which will. Remember for limit comparison, we take the expressions in the two series we are comparing and form the ratio.
State which test you are using, and if you use a.